
The authors discuss how to develop a cost-effective thermally protective packaging system.

The authors discuss how to develop a cost-effective thermally protective packaging system.

Vetter is investing $100 million to expand filling capacity in Germany and the United States.

ATMI has invested in sterile-connector and sterile-fill technology developed by Medical Instill Technologies.

FDA Issues Draft Guidance on Patient Counseling Info for Labeling

As the Supreme Court ruled on generic-drug liability, FDA outlined new rules for warning labels.

Vetter has ready-to-submit documentation for this service in Common Technical Document (CTD) formats for the US, Europe and Japan.
A Q&A with SCHOTT Pharmaceutical Systems and West Pharmaceutical Services
An integrated focus on product design, development, operation, and control.

New design meets new process requirements.

Prefilled-syringe line features automation and novel disinfection techniques.

Aggregate formation is influenced by multiple aspects of the bioproduction process but can be mitigated by good process design and control.

NIBRT's Ray O'Connor provides an overview of aseptic processing.

The European Commission (EC) is seeking to introduce a black symbol to identify medicines that are subject to additional monitoring.

New US Pharmacopeial Convention (USP) standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.

Ray O'Connor, an operations consultant with NIBRT, addresses aseptic processing, including how to avoid contamination, and cleanroom best practices. Posted May 2012.

FDA has released a final guidance describing cGMP for preparing media fills for validation of aseptic preparations for positron emission tomography (PET) drugs. Most PET drugs, which are used for imaging, are given parenterally, and produced by aseptic processing. PET drugs came under the auspices of FDA relatively recently, with the passage of the 1997 Food and Drug Administration Modernization Act, and minimum cGMP standards for this class of drugs were established in 2009. A guidance for preparing new drug applications and abbreviated new drug applications for PET drugs was issued in March 2011, and in comments to the March 2011 guidance and in questions raised at the public meeting, stakeholders requested that FDA provide guidance on media fills for validation of aseptic preparation for PET drugs. This most recent guidance is designed to help manufacturers of PET drugs comply with FDA regulations.

A technical rountable featuring Sartorius Stedim Biotech, Pall Life Sciences, 3M Purification, Asahi Kasei Bioprocess, and Bio-Rad Laboratories.

The authors describe a new assembly for bulk and final drug product filling operations.
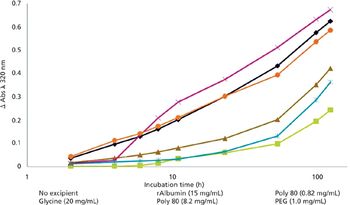
Recombinant albumin can stabilize a drug product and assist in API release.
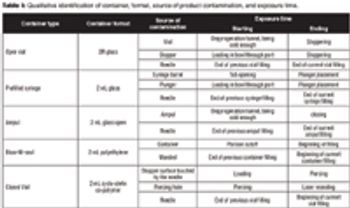
The authors compare the exposure risk from viable particles from the air supply in four well-established aseptic filling technologies.

The International Society for Pharmaceutical Engineering (ISPE) will soon publish an update for its guide to sterile-product manufacturing facilities. The new publication will replace the original guide, ISPE Baseline Guide: Sterile-Product Manufacturing Facilities, and contain practical information about technological advances in sterile manufacturing.

The author looks at strategies to minimize particle levels in the finished product when using single-use technologies downstream of final capabilities.
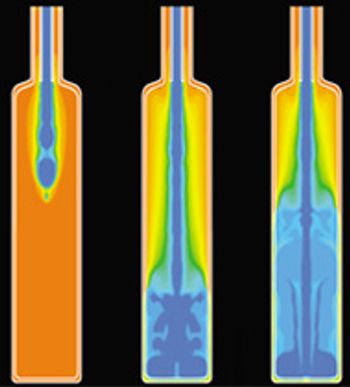
The authors give special consideration factors affecting blow–fill–seal technology.

FDA, NIH, and industry seek new strategies to spur drug development and promote access to therapies.

Genzyme has signed a new supply agreement with Hospira Inc