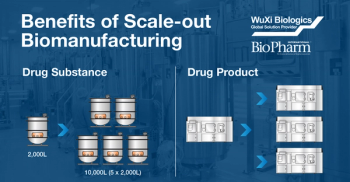
Perfusion technology is a tool to increase productivity that can be applied at different stages during upstream cell culture for biotherapeutics products. Perfusion allows removal of waste by-products and replenishment of fresh nutrients so that the cells are able to grow and reach higher cell densities and higher productivity. N-1 perfusion, the application of the perfusion technology in the step immediately before the production bioreactor, is a good option for processes that exhibit low productivity. Join Claudia Berdugo, Ph.D., Director of Process Development at Catalent Biologics, for a podcast where she discusses N-1 perfusion and how Catalent Biologics works toward process improvements for each client to enable them to move their pharmaceutical products forward with confidence.