
Manufacturers challenge details in new policies designed to promote access to important therapies.

Manufacturers challenge details in new policies designed to promote access to important therapies.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

According to AstraZeneca, the purchase of the biologics bulk plant will double the company's biologics manufacturing capacity in the US.

FDA announced the recall, citing deficiencies in Medistat’s aseptic processing areas and in its environmental monitoring procedures.

Wilden’s Saniflo Hygienic Series pumps use a new, energy-efficient air distribution technology.
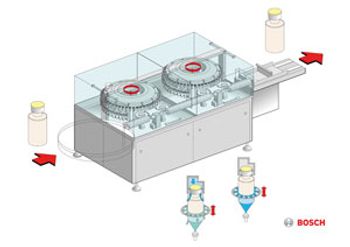
The RAN 3080 exterior washing machine from Bosch cleans filled and closed vials, ampuls, and cartridges using a high-pressure process and special transportation system.

Aragen Bioscience has licensed ProteoNic Biotechnology’s 2G UNic recombinant protein production technology, which increases manufacturing efficiency and reduces cost of goods for recombinant biologicals.

FDA warns that compounded or repackaged drugs stored in certain syringes made by Becton-Dickinson may lose potency because of an interaction with the rubber stopper.

FDA publishes the final Q3D Elemental Impurities guidance.

The Biosimilars Forum argues that the assignment of a unique Healthcare Common Procedure Coding System (HCPCS) for each biosimilar product is in alignment with the intent of Congress.

The agency has set up a workshop on how to demonstrate the benefits of orphan drugs over existing treatments.

The agency releases guidance on the nonclinical evaluation of endocrine-related drug toxicity.

The agency releases guidance on good review practice for formal dispute resolution in regards to appeals.

The European Medical Contract Manufacturing (EMCM) organization announced it will team up with VCC Medical NV for a unique personalized medicine initiative-the aseptic manufacture of a biologic therapeutic from the tissue of a patient’s tumor.

Sandoz announced on Sept. 3, 2015 that despite the barriers erected by competitor Amgen, Sandoz officially launched Zarxio (filgrastim-sndz), its biologic version of Amgen’s neutropenia medication Neupogen (filgrastim). To complement the drug launch, Sandoz is also launching Sandoz One Source, a patient services center providing support for and information on the medication.

Reuters reports that Novo Nordisk will invest 500 million Danish Krone (approximately $75 million US dollars) into a new 19,000 m2 warehouse in Hillerød, Denmark. The warehouse will handle all inbound raw materials for Novo Nordisk's production in Denmark and will have a capacity of around 17,000 pallets of product, according to the news outlet.

The investigational monoclonal antibody targets interleukin-17 and is in clinical trials for the treatment of plaque psoriasis.

The platform, developed by Vectron Biosolutions, will be used for BI’s in-house pipeline and for its outsourcing clients.

The collaborative effort will be focused on fully humanized antibodies.

Under the terms of the agreement, Sartorius Stedim Biotech will manufacture membrane adsorber technologies for GE, which will be marketed as part of GE’s ReadyToProcess product offerings.

UPS joins the Global Health Supply Chain Technical Assistance program to help secure the drug supply chain.

When should CMOs and their pharma clients share the details of their partnerships with outside parties?

The agency started the full operation of its medical literature monitoring on Sept. 1, 2015.

Adents’ Pharma Suite serialization software features track-and-trace capabilities.

Pharma scientists announced for AAPS executive council and section leadership positions.

Growth is said to be driven by the deeper penetration of biosimilars in developed and emerging markets as a result of clearer regulatory pathways.

The drug was the first PCSK9 inhibitor approved in Europe and the second approved in the US.

GSK announces plan to divest its rights in ofatumumab for auto-immune indications for up to $1 billion.


The TRUST project will focus on the process development of Col-Treg, which is TxCell’s autologous collagen type II specific Treg immunotherapy product for the treatment of autoimmune uveitis.