
FDA grants support US research in continuous manufacturing monitoring and control techniques for bio/pharmaceutical manufacturing at Rutgers, MIT, and Georgia Tech.

FDA grants support US research in continuous manufacturing monitoring and control techniques for bio/pharmaceutical manufacturing at Rutgers, MIT, and Georgia Tech.

FDA sent a warning letter to Apotex Research Private Limited after investigators found current good manufacturing practice violations.

The companies entered a multi-year R&D collaboration to develop mRNA-based flu vaccines.

FDA published a resource guide to promote responsible opioid prescribing in the treatment of animals.

Pharmaceutical scientist association announces upcoming term’s board of directors.

Drug product approval from FDA follows previous approvals from European and Japanese authorities.

The new drug, Onpattro (patisiran), by Alnylam Pharmaceuticals, is in a new class of drugs called small interfering ribonucleic acid (siRNA) treatment.

The agency issued a warning letter to Canadian API manufacturer, Les Produits Chimiques B.G.R, citing cGMP violations at its API facility in Pointe-Claire, Quebec.
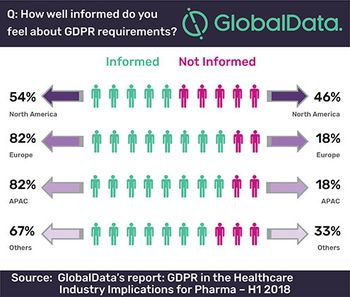
Only 54% of North Americans feel informed about the requirements of the general data protection regulation (GDPR), according to a report by GlobalData.

The Alliance for Regenerative Medicine’s (ARM) report highlights major trends and metrics from the 2018 second quarter in the regenerative medicines sector.

Ebola vaccinations by the World Health Organization began in North Kivu, Democratic Republic of the Congo, one week after the country’s latest outbreak.

The combined companies will provide research- and clinical-grade human immune cells, blood products, and related services.

The European Medicines Agency (EMA) will temporarily scale back activities as it copes with “significant staff loss” and prepares for the next phase in its continuity plan.

Avalon and Weill will co-develop bio-production and standardization procedures for CAR-T therapy.

The European Commission (EC) approved Pfizer’s Trazimera (trastuzumab), a biosimilar to Roche’s Herceptin, to treat certain breast and gastric cancers.

The companies will collaborate on the discovery, development, and commercialization of cell therapies for cancer.

Boehringer Ingelheim joins Oxford BioMedica, UK Cystic Fibrosis Gene Therapy Consortium, and Imperial Innovations to form a partnership for developing a new gene therapy to treat cystic fibrosis.

AuroMedics Pharma issued a voluntary, nationwide recall of two lots of piperacillin and tazobactam for injection, USP 3.375 g due to the presence of particulates identified as glass and silicone material.

The companies will collaborate to bring InnoCore Pharmaceuticals’ proprietary SynBiosys biodegradable polymer platform to market.

Researchers at the University of Pennsylvania found that diabetes drugs called thiazolidinediones can promote the metabolism of glutamine to help control disease-causing inflammation.

Stem-cell developer TiGenix has been acquired by Takeda Pharmaceutical for approximately EUR 520 million (US$604 million).

The company broke ground on its $200-million, 120,000-ft2 biomanufacturing plant in West Greenwich, RI.

GlaxoSmithKline and 23andMe, a personal genomics and biotechnology company, will partner to research and develop new drugs using human genetics.

The companies will work together to develop novel gamma delta T-cell receptor therapies in various cancers.

Frustrated by slow market adoption, Gottlieb maps out new plan for biosimilar competition.

Novartis is set to partner with MorphoSys and Galapagos in a deal worth up to $1.1 billion to develop and commercialize their joint investigational, fully human, IgG1 monoclonal antibody (mAb) directed against the target IL-17C.

Pfizer will invest nearly half a billion dollars to build a sterile injectable facility in Michigan.

The recommended drugs include two orphan medicines and three biosimilars.

The National Science Foundation grant will be used to commercialize a synthetic biology platform for cancer drug development.

Quartzy, an online laboratory supply management company, will offer lab products from Bioline, Biotium, and MP Biomedicals.