
The authors investigate the sufficiency of high-temperature short-time treatment in inactivate mouse minute virus contamination.

The authors investigate the sufficiency of high-temperature short-time treatment in inactivate mouse minute virus contamination.

The authors review the technologies that may help bioprocessing become a truly continuous operation and present case studies that could contribute to the integration of upstream and downstream platforms.
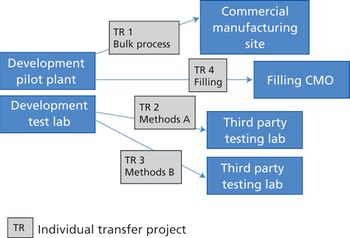
Careful planning, adequate staffing levels, and experienced project managers can help avoid pitfalls of transferring processes from one facility to another.
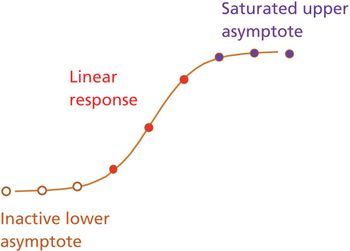
The author describes common components of a relative potency bioassay and provides a framework for assay development, calculation, and control.

Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

Selecting a delivery method early on may be beneficial.

A modular cell-culture platform demonstrates accelerated process development.

Proven science-based strategies can help to accelerate vaccine development.
The authors provide application data to support the use of SEC beyond small-scale operations.

Global outbreaks energize vaccine R&D and drive production modernization.

Demand is driving expansion and consolidation of formulation and clinical trial materials services.

Industry experts provide insights on the challenges and importance of using buffers in downstream processing.

Click the title above to open the BioPharm International April 2016 issue in an interactive PDF format.