
As the Supreme Court ruled on generic-drug liability, FDA outlined new rules for warning labels.

As the Supreme Court ruled on generic-drug liability, FDA outlined new rules for warning labels.

Pharma and biotech companies, with the rest of the healthcare industry, must face change.

Ranbaxy's $500 million settlement for producing adulterated drugs and fradulent data provides a cautionary tale for patients, FDA, and drug manufacturers.

Wanted: Aspiring authors to share technical and scientific solutions for biopharmaceutical processing.

Sound policies are needed to govern the substitution of interchangeable biologics.

A QbD paradigm advances process understanding in development and manufacturing.

Ever since Twitter launched in 2006 and Facebook became mainstream, most industries have sought ways to connect with their consumers through social media.

Can postapproval FDA filings immunize pharma companies from patent lawsuits?
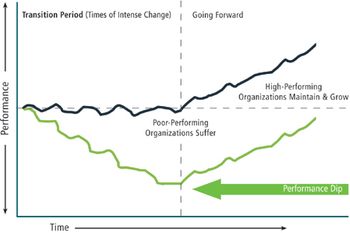
A disciplined approach to changing behavior can achieve change agility.

Do you have to worry about FDA releasing confidential data? Apparently so.

Working together affords many unseen opportunities for pharmaceutical innovation.

Ties between the biotechnology industry and university research are crucial.

PDA's strategic plan calls for maintaining valuable relationships with global regulators.

Now that the Supreme Court has upheld the Affordable Care Act, what's next for biopharma?

BIO is calling for a more patient-centric approach to user-fee reauthorization.

Formulators and developers are at the heart of the industry's basic premise-they are saving lives.

Future sponsor-contract provider relationships will require more integration.

Rather than seeking a single indication for one large group, the orphan-drug approach segments the market for a drug more minutely.

Collaboration can begin with a conversation.

The confluence of science, technology, and regulation will provide our industry with the guidance to move forward.

Does global development have to entail multiple comparability studies?

New educational programs are key to the industry's future and to safe, available drugs.

Government plans require investment, partnership, and industry collaboration.

Reauthorization of pediatric exclusivity provisions looms in 2012 and debates begin anew.

With the rise in therapeutics comes more complex partnerships.