
Bioproduction - Achieving Quality by Design through Statistical Process Control

Bioproduction - Achieving Quality by Design through Statistical Process Control
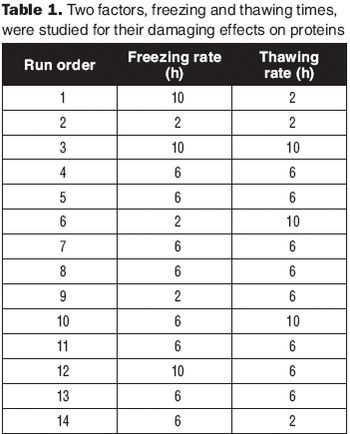
Apply a DoE strategy to test several formulations in parallel.

Evaluate and communicate risk to stakeholders.
Adequate characterization of materials protects product quality.

Industry and regulators disagree over noncritical parameters.
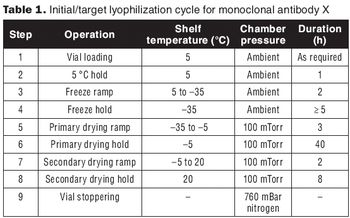
Develop a relevant design space without full factorial DoE.
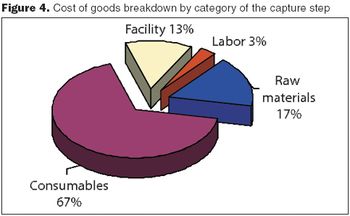
Combine cost analyses with QbD to improve operations and lower costs.


One might look at QbD's plodding growth and conclude that it is never going to make it to graduation.

How will implementing Quality by Design strategies affect your compliance status?

The nimbleness of biotechs makes them well suited to implementing QbD. Here's how to get started.

Regulatory flexibility can make continuous improvement possible.

QbD has always embraced the notion that companies could make certain process changes without regulatory oversight.
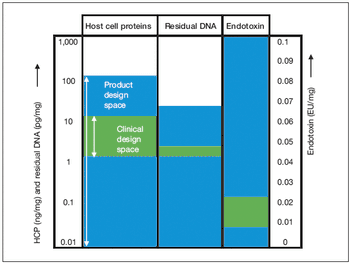
Key considerations for defining your overall control strategy.

When the CMC Biotech Working Group began developing a case study on applying Quality by Design (QbD) to biotech products, the goal was to challenge conventional thinking on the subject.
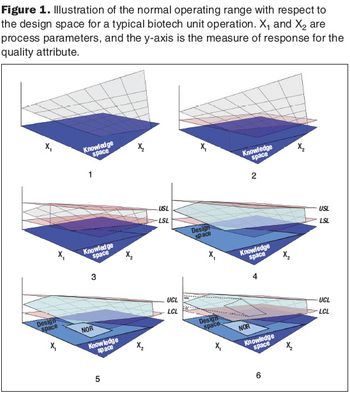
Second in a three-part series that discusses the complexities of QbD implementation in biotech development.
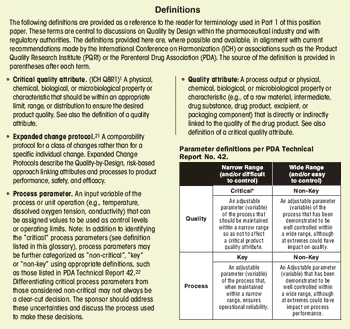
First in a three-part series that discusses the complexities of QbD implementation in biotech product development.

The focus on the design space will lead to a new workspace, and will affect staff in the development, manufacturing, and quality functions.

Without a rigorous discussion of the pros and cons of QbD, its tremendous benefits will be lost.

When the final version of the Quality by Design (QbD) case study is made public this summer, it will be an aspirational document, says Ken Seamon, PhD, one of the project?s facilitators. "If the regulatory authorities read our final document and said 'yes, this is all fine,' we will have failed," he said.

QbD for QA? Try a two-step approach

Understanding the relationship between the process and CQAs.

No time for QbD? How to convince management to make it a priority.

The FDA's revised process validation guidance manages to explain the underlying concepts of Quality by Design without every using the phrase.
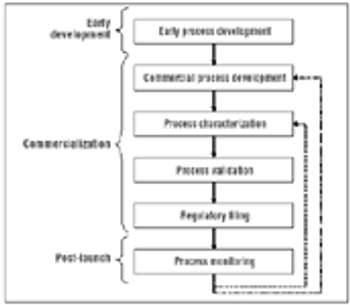
Using multivariate experiments to define acceptable ranges.