
CDMOs and CMOs face weak economic recovery, consolidation, and globalization.

CDMOs and CMOs face weak economic recovery, consolidation, and globalization.

Covance's deal with Sanofi-Aventis demonstrates the power of scale and scope.

Venture capital is still scarce for early biotechs and their providers.

How current economic conditions affect your build-or-buy decision.

This month, we catch up on major developments around the world and their implications for the contract services industry.
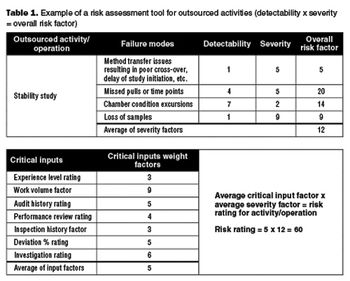
Apply risk management principles to monitor outsourced activities.

How much to spend on early development, whether to use your CMO's proprietary cell line, and other outsourcing advice.

Being open to unforseen events may avert risk or create opportunities.
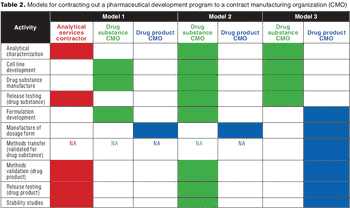
Formulation strategy is an important consideration when selecting and managing outsourced biopharmaceutical development programs.

It is important that all stages of the audit be given an equal measure of attention.
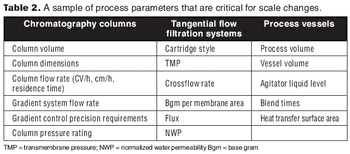
A process harmonization assessment can aid in smooth technology transfer by comparing data across equipment and sites.

Insourcing is new outsourcing. Here's how to do it right.

Partner with a contract manufacturing organization that integrates best practices from project management, customer service, and Lean.

The ability to deal with the complexity of the clinical supply process has shifted the balance of power to clinical supply chain specialists.

Collaborations between Western and Indian companies may provide the best path for offshoring successfully and for developing India's readiness.
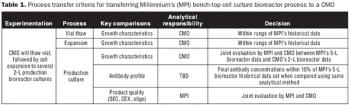
Sufficient process history is key to the rapid transfer of your process.

As facility closures and layoffs continue, CRO and CMO executives are hoping for a better year, despite few positive signs.

A systematic classification system makes supplier quality management feasible, even if you are dealing with hundreds of suppliers worldwide.

A new analysis highlights growth opportunities and challenges for contract development and manufacturing organizations.

An enterprise-wide quality management initiative is required to maintain supplier quality without sacrificing bottom-line objectives.

Lonza's bid for Patheon makes the contract manufacturing industry re-examine the one-stop manufacturer model.

To assess current trends in outsourcing, BioPharm International turned to Mark Douglas, PhD, strategic business development manager, Avecia Biologics Ltd; Geoffrey M. Glass, senior vice president of strategy, corporate development and business integration, Patheon Inc.; Rob Gustines, director of marketing, KBI Biopharma, Inc.; Jon S. Kauffman, PhD, director, method development & method validation and biopharmaceutical services, Lancaster Laboratories; Roger Lias, PhD, president, Eden Biodesign, Inc.; John A. McCarty, vice president, formulation sciences and drug delivery, Azopharma; and Michiel E. Ultee, PhD, vice president, process sciences, Laureate Pharma, Inc.

To assess current trends in outsourcing, BioPharm International turned to Mark Douglas, PhD, strategic business development manager, Avecia Biologics Ltd; Geoffrey M. Glass, senior vice president of strategy, corporate development and business integration, Patheon Inc.; Rob Gustines, director of marketing, KBI Biopharma, Inc.; Jon S. Kauffman, PhD, director, method development & method validation and biopharmaceutical services, Lancaster Laboratories; Roger Lias, PhD, president, Eden Biodesign, Inc.; John A. McCarty, vice president, formulation sciences and drug delivery, Azopharma; and Michiel E. Ultee, PhD, vice president, process sciences, Laureate Pharma, Inc.

To assess current trends in outsourcing, BioPharm International turned to Mark Douglas, PhD, strategic business development manager, Avecia Biologics Ltd; Geoffrey M. Glass, senior vice president of strategy, corporate development and business integration, Patheon Inc.; Rob Gustines, director of marketing, KBI Biopharma, Inc.; Jon S. Kauffman, PhD, director, method development & method validation and biopharmaceutical services, Lancaster Laboratories; Roger Lias, PhD, president, Eden Biodesign, Inc.; John A. McCarty, vice president, formulation sciences and drug delivery, Azopharma; and Michiel E. Ultee, PhD, vice president, process sciences, Laureate Pharma, Inc.

To assess current trends in outsourcing, BioPharm International turned to Mark Douglas, PhD, strategic business development manager, Avecia Biologics Ltd; Geoffrey M. Glass, senior vice president of strategy, corporate development and business integration, Patheon Inc.; Rob Gustines, director of marketing, KBI Biopharma, Inc.; Jon S. Kauffman, PhD, director, method development & method validation and biopharmaceutical services, Lancaster Laboratories; Roger Lias, PhD, president, Eden Biodesign, Inc.; John A. McCarty, vice president, formulation sciences and drug delivery, Azopharma; and Michiel E. Ultee, PhD, vice president, process sciences, Laureate Pharma, Inc.