
The benefits of harmonization may be on industry's wish list, but buying into change is another story.

The benefits of harmonization may be on industry's wish list, but buying into change is another story.

Government plans require investment, partnership, and industry collaboration.

Greater emphasis on focus and efficiency for companies as market demands value in 2012.

Political leaders need to consider the impact of the biopharmaceutical industry on the economy.

With the rise in therapeutics comes more complex partnerships.
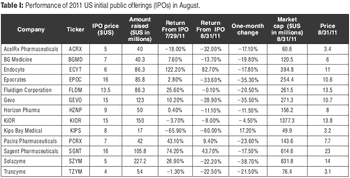
Volatile Markets Take a Toll on Life-Sciences Initial Public Offerings

Inside the National Institutes of Health

Market considerations and new technologies must be recognized to achieve the full benefits of manufacturing prefilled syringes.
An interview with Oskar Gold, vice-president, key account management and corporate marketing, at Vetter.
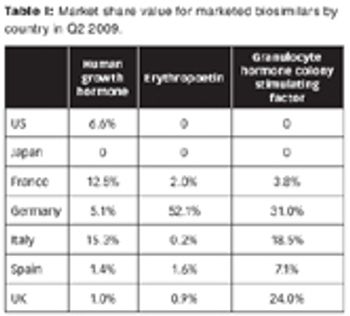
The market landscape for biosimilars is in flux, with limited penetration now, but with the potential for growth for those who can navigate the market. Plus: A SWOT analysis of biosimilars by Anjan Selz.

FDA weighs multiple views regarding the Biologics Price Competition and Innovation Act.

Industry may be its own obstacle to success in achieving the desired high-performance state.

Are biosimilars the next big thing or just the next big bubble?
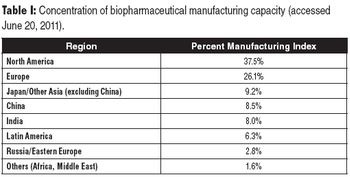
Insights from real-time ranking of global biomanufacturing capabilities.

Which route will we take to arrive at a national stem-cell policy?

Strong pipelines, approvals, and deals drive up market cap.
BIO's president and CEO provides insight leading up to the 2011 convention.

Patent databases are often overlooked as a source of basic science, research, and development

Knowing where key biomanufacturing facilities are located around the world is essential for decision-making.

Those who doubt there's faith in science, should check out our annual Bioprocessing Survey.

Two films portray, in surprising detail, the hard work and determination of the biopharmaceutical industry.

Research and development headed for divorce.

Industry starts the year with a positive spin.

Those at the top must walk the walk of uncompromising commitment to compliance
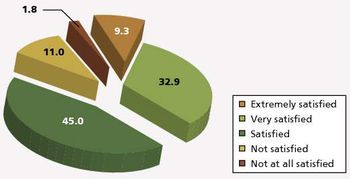
How did biopharmaceutical professionals fare during a year of hiring freezes, high anxiety, and increased workload? Our survey finds out.