
Podcasts


Imagine a world where cleanroom facilities—essential for pharmaceutical manufacturing, biotechnology, and high-tech industries—are built with unparalleled speed, precision, and efficiency. That world is here, thanks to the hybrid construction approach. By blending traditional stick-built methods with modular and prefabricated solutions, companies are overcoming the limitations of conventional construction while ensuring compliance with stringent industry regulations. In this interview, we explore how hybrid cleanroom construction is transforming the industry, offering faster project timelines, improved quality control, and significant cost advantages. Join us as we delve into this game-changing approach with industry experts who are leading the charge in revolutionizing cleanroom infrastructure.

In this episode, we explore BIOVECTRA’s capabilities in antibody-drug conjugate (ADC) manufacturing, from complex conjugation chemistry to synthesis of highly potent payloads. We’ll also showcase how BIOVECTRA’s extensive experience in complex chemistries and specialized small molecule manufacturing gives them a unique perspective, strengthening their approach to ADC production and ensuring clients receive custom solutions across all project stages.

Metabolic Flux Analysis (MFA) is a powerful technique used to characterize metabolic phenotype driving to improved productivity in biomanufacturing. MFA is most powerful when absolute concentrations of the metabolic intermediates are measured, but doing so in practice is often impractical due to inherent limitations of conventional absolute quantitation by mass spectral analysis. Recently, advances in artificial intelligence (AI) have been applied to solving the problem of broad, untargeted, absolute quantitation in liquid chromatography-mass spectrometry (LC-MS). These new approaches extend readily to the determination of absolute concentrations of stable isotopically-labeled metabolic intermediates, offering a new tool for MFA. Dr Sam Yenne from Metalytics Inc., a company that specializes in the science of metabolic flux, has recently assessed and adopted Pyxis, a new tool for absolute quantitation of raw LC-MS data. Dr Yenne describes MFA, its utility in biopharmaceutical development, associated challenges and how Pyxis impacts those challenges.

The nexus between biopharmaceuticals and sustainability is seemingly far apart, however, it is increasingly recognized as an inevitable challenge. It is encouraged to take a sustainable approach to reducing the environmental impact of the production and supply of medicines while improving people's health; delivering the well-being of people and the planet. Yosuke Shimojo (Technical Value Support Section Manager, Nagase Viita) will unveil how SOLBIOTE™, a portfolio of injectable-grade saccharide excipients, would be a key for the biopharmaceutical development and achieving sustainability for a better future of the industry.

This podcast explores the challenges of and the progress made so far by the biopharma industry toward alternative drug delivering methods for biologic drugs.

In this podcast, we will explore the advantages of partnering with a Contract Development and Manufacturing Organization (CDMO) to navigate the journey from molecule conception to clinical trials, including the expertise, experience, and preparedness that a CDMO brings to the table. The audience will gain valuable insights into crucial questions such as the optimal stage in the development process to engage with a CDMO, the necessary preparations for a successful collaboration, and whether a finalized molecule is required to initiate discussions with a CDMO. Join Curia’s Director, Process Development Science, Scott Alderucci, who leads the process development group at Curia, a global CDMO offering CDMO expertise for biologics and small molecules.

Robust, scalable manufacturing of novel therapeutics requires partnering with a CDMO equipped to adapt to changes in the market and knowledgeable on the latest technologies used to support cell line development.

Bernhard Schlichtner, Chief Commercial Officer at Single Use Support, offers valuable insights into why adopting a holistic approach to process solutions is the key to successful fluid & cold chain management.

Intensified continuous chromatography, a type of Multi-Column Chromatography (MCC), has emerged as a valuable technique in downstream bioprocessing that enables increases in productivity. In this podcast, MCC Product Specialist, Jennifer Knister, will discuss how adopting MCC can reduce costs, decrease suite time, and lead to higher resin utilization, along with why these benefits are particularly worthwhile in multi-product facilities.

Biopharma Resilience is declining. The 2023 Global Biopharma Resilience Index from Cytiva offers insights into the current state of the industry, shining a light on both areas of strength and opportunities for collaborative growth. By working together, biopharma leaders, academia, and policymakers can create lasting improvements. Join Ludovic Brellier, President of Biotechnology Integrated Solutions and Business Operations Leader at Cytiva, as he delves into this year’s Index findings. Specifically, he’ll highlight where the industry should turn their focus to ensure that patients are continuing to get the essential life-changing therapies they need.

Hanns-Christian Mahler and Andrea Allmendinger from ten23 health will discuss some key aspects of biologic drug development and manufacturing.

Creating a new therapeutic drug can take a decade or more to get to patients. Antibody production and discovery play an integral role in setting the stage for success at later stages of development. If a high affinity, functional antibody candidate is discovered early, the timeline to a finished targeted and effective therapeutic drug may be significantly reduced. In this podcast we will go through the untold tales of antibody production and discovery, as well as how Twist has optimized a process for antibody production and screening for potential therapeutics.

Adeno-associated virus (AAV) is a widely used viral vector for the delivery of genetic material in gene therapy. Vector development and production at an industrial scale requires an efficient method for accurate quantification of viral capsids. Unfortunately, traditional techniques such as ELISA, HPLC, and ddPCR are time consuming and labor-intensive. This podcast introduces a rapid, label-free method for the direct capture and quantitation of various AAV serotypes using the Octet® Bio-Layer Interferometry (BLI) platform with Octet® AAVX Biosensors. These biosensors deliver results in as little as 15 minutes and can be used to quantitate AAV capsids in both crude and purified samples.


In this interview we will discuss recent strategies for upstream bioprocess analytics and how a thorough process understanding can improve product quality and process efficiency.




Multi-column continuous chromatography (MCC) offers significant economic advantages over traditional batch methods for the purification of monoclonal antibodies (mAbs), including increased resin capacity utilization, smaller columns, reduced buffer consumption, and faster processing time. For mAb productivity, the Protein A capture step is a primary target to apply MCC due to its high cost, which is driven even higher as improvements in upstream processing have produced a steady increase in mAb titers. The multi-column continuous chromatography method has demonstrated that mAb productivity can be increased by 400 to 500% compared to batch-mode.

For years pharmaceutical and biopharmaceutical manufacturers have invested in rigid stainless-steel equipment for upstream bioprocessing. With new and innovative single-use products now on the market, manufacturers have access to flexible, safe solutions that offer a myriad of advantages over the traditional solutions. Single-use technology offers less risk of contamination, more operator safety, and less negative environmental impact than the stainless-steel alternatives. In this podcast, we will discuss the challenges associated with handling powders in upstream biopharm processes and how single-use technology can benefit manufacturers from an environmental, cost, and safety perspective.

This is the year in which manufacturers will have the courage to bet on data aggregation and AI solutions that help them grow their capacity, moving away from pilot mode and embedding those tools as foundational assets. These have become critical levers to support the expanded demand for drug manufacturing, especially exacerbated with the pandemic. Join John Vitalie, CEO at Aizon, as we discuss how Pharma 4.0 is transforming the pharma landscape to bring gains to manufacturers and to the patients they serve.

Fill finish (sterile filtration) operations are critical and often the only means to produce sterile liquid biopharmaceuticals as other means of sterilization will degrade the product(s). This complex packaging process has become more regulated and controlled in an effort to reduce the risks of contamination (with the greatest risk potential coming from cleanroom operators). This episode reviews current market trends and provides some characteristics expected of a top notch Fill Finish CMO, like Samsung Biologics in Incheon, South Korea.

It is the bottleneck in pharmaceutical manufacturing: Lead times. Which are the approaches that may secure business continuity? What are ways to overcome dependencies on vendors’ supply? Barbara Fischer gives insights on how single-use system providers tackle this industry-wide challenge in supply chain.
Lipid-based formulations are gaining global awareness, notably with lipid nanoparticles (LNPs) being a key component in several current COVID-19 and mRNA vaccines. Lipid-based formulations, including liposomes and LNPs, are an effective drug delivery system for a variety of active pharmaceutical ingredients (APIs), notably for complex parenteral drug products including cancer, viral, and gene therapeutics. In this podcast, we discuss how Emergent has been involved in the manufacturing of lipid-based formulations over the past two decades, along with factors to consider when partnering with a CDMO for lipid-based formulation manufacturing.

Perfusion technology is a tool to increase productivity that can be applied at different stages during upstream cell culture for biotherapeutics products. Perfusion allows removal of waste by-products and replenishment of fresh nutrients so that the cells are able to grow and reach higher cell densities and higher productivity. N-1 perfusion, the application of the perfusion technology in the step immediately before the production bioreactor, is a good option for processes that exhibit low productivity. Join Claudia Berdugo, Ph.D., Director of Process Development at Catalent Biologics, for a podcast where she discusses N-1 perfusion and how Catalent Biologics works toward process improvements for each client to enable them to move their pharmaceutical products forward with confidence.
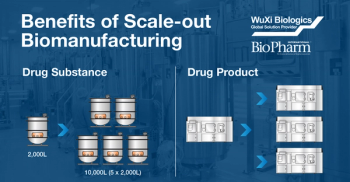
Changes in production scale required due to increasing product needs throughout clinical trials introduces risk to the product development timeline. Both the process and the product can be dramatically impacted from scaling-up to larger production volumes. Once in commercial manufacturing, sudden changes to product demand in either direction can have costly consequences if the scale of production chosen is not adequate to meet the changing material requirements. Adapting a “scale-out” instead of “scale-up” strategy for clinical and commercial production can greatly minimize these risks. This podcast will review the scale-out biomanufacturing approach and the significant benefits of this production paradigm.
The past few years have been filled with seminars discussing viruses, with COVID-19 stealing most of the headlines. Not all viruses are created equal; some are actually beneficial for human health and biotherapeutics delivery. Adeno-associated virus (also referred to as AAV) vectors fall into this category. AAVs are “hot” right now, as they are currently one of the most promising systems for human gene therapy. There are many types of AAVs and purification and analysis of these types of viruses can present challenges. In this dedicated dialogue, we will discuss the most efficient workflow for AAV purification and analysis.

Choosing the right sterilization technique for your drug product and its packaging is not always a simple decision. While most packaged drug products have specific requirements, newer sterilization techniques offer innovative ways of ensuring sterility that may be more suitable than standard sterilization processes. In this dedicated dialogue, the audience will learn about standard sterilization processes, be introduced to newer techniques, and understand how these techniques have been applied to parenteral packaging components.

Astrea Bioseparations joins Biopharm International for a discussion about the evolving challenges of downstream processing for advanced therapies, and what the future might hold. We talk about the company’s evolution over the last three decades, including the Discover. Develop. Deliver. approach to customer alignment. The latest industry trends are reviewed, along with considerations to keep in mind during the selection of off the shelf or customized downstream solutions.