Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?

Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?

Industry experts discuss best practices for selecting a separation technology.

Advances in cell culture media technology have helped achieve safer biologics.

The BioProfile FLEX 2 from Nova Biomedical is a cell-culture chemistry analyzer that allows for automated cell-culture analysis for small-volume culture systems.

The OM-CP-CRYO-TEMP ultra-low temperature data logger from Omega Engineering records temperatures as low as -86 °C.
This study offers a strategy for stabilization of biotherapeutics for long-term frozen storage in PBS-based formulations.

Airlines, airports, freight forwarders, and other cold-chain partners are taking a crash course in pharma cGMPs.

Model effectiveness is determined by the quality and composition of the data inputs.
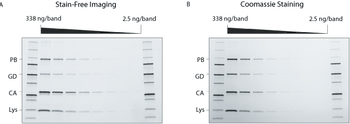
Innovations in electrophoresis and chromatography upstream of protein characterization can accelerate research.

Manufacturers and regulatory authorities seek coordinated lifecycle management policies.

The strategies of a innovation-driven CMO may be different than a capacity-driven CMO.

Click the title above to open the BioPharm International September 2016 issue in an interactive PDF format.

The Parenteral Drug Association develops program to address barriers to implementation of postapproval changes.

Steep price increases for a popular drug have created patient and Congressional backlash.