
Top 1000 Facilities

Top 1000 Facilities
BIO's president and CEO provides insight leading up to the 2011 convention.
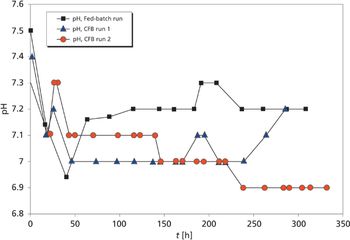
Cell concentrations and resulting protein concentrations are higher in a concentrated fed-batch process than in a standard fed-batch culture system.
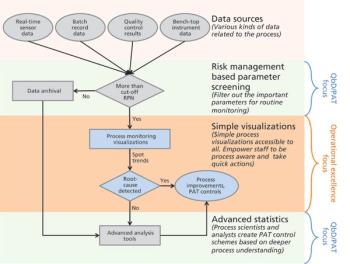
The authors focus on operational excellence in manufacturing of biotechnology therapeutic products in the QbD paradigm.

Single-use conference roundup.
The authors present a case study identifying a contaminant.

A recent industry survey shows keen interest in improving bioreactors and cell-culture media.

Industry struggles to curb drug abuse, diversion, and disruptions in supply to ensure access to quality products.

Patent databases are often overlooked as a source of basic science, research, and development

Knowing where key biomanufacturing facilities are located around the world is essential for decision-making.
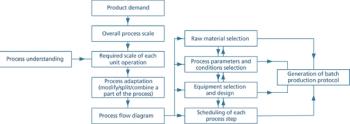
Clear documentation and open communication are essential for effective technology transfer.