
Line of Environmental Measurement Products

Line of Environmental Measurement Products

UPLC Separation Intergrates into Mass Spectrometers
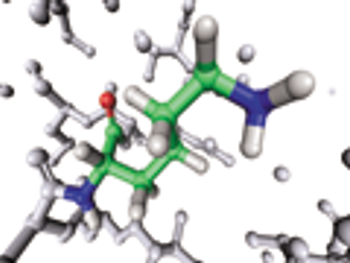
USP evaluates raw materials used in the chemical synthesis of peptides.

New identifiers and tracking requirements aim to block illegitimate products.
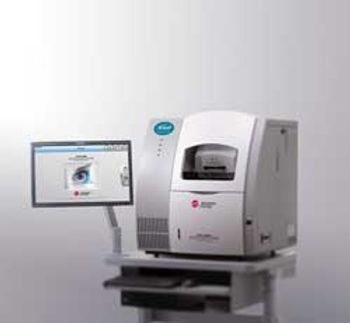
High-Performance Separation-Electrospray Ionization System

Life-sciences companies continue upward momentum in 2014.

How badly is Brazil's pharmaceutical market suffering from the global instability of emerging markets?
The biopharmaceutical industry is developing a new approach to controlling variability in raw materials.
The approval and acceptance of monoclonal antibody biosimilars is necessary if the biosimilars market is to experience real growth.

More media options open publishing opportunities for bioprocessing experts and authors.

Outsourcing activity remains strong and unlikely to abate, especially in more traditional areas.

Mass Spectrometer Enhances Performance in Protein Analysis
Liquid Chromatography Column Separates Glycans

Aggregation System Improves Analysis of Sub-Visible Particles
The presence of minute amounts of chelators can help minimize the degradation of monoclonal antibodies.

As Europe strives to firmly incorporate quality-by-design principles, there are several key issues that still need to be addressed.

Click the title above to open the BioPharm International April 2014 issue in an interactive PDF format.