
Potential interference with maternally-derived antibodies makes most vaccines less effective in the neonate.

Potential interference with maternally-derived antibodies makes most vaccines less effective in the neonate.
Human HBV can be grouped into eight genotypes, which differ by at least 8%.
Microstructured transdermal systems can deliver a vaccine in close proximity to the antigen-presenting cells in the epidermis.

The approval of a new seasonal influenza vaccine has further diversified the supply to the US market.

The US Food and Drug Administration has approved the use of the nasal influenza vaccine FluMist in children ages 2?5.

Sanofi Pasteur (Lyon, France) has announced positive results from Phase 2 trials of its dengue vaccine candidate based on technology licensed from Acambis plc (Cambridge, UK).

Crucell N.V. (Leiden, the Netherlands) has discovered a set of human monoclonal antibodies (MAbs) that work to neutralize and protect against H5N1.

Sanofi Pasteur (Lyon, France) has produced data showing that its new investigational H5N1 pandemic influenza vaccine containing a proprietary adjuvant achieved a high immune response at the lowest dose of H5N1 antigen reported to date.

Five European organizations, including three companies and two hospitals, have joined forces to develop a novel pandemic influenza vaccine as a potential emergency vaccination.

It is hoped that HIV patients' own immune responses can be strengthened by vaccines so they will not have to rely exclusively on antiretroviral drugs.
For pandemic vaccine processing, single-use filter cartridges and membrane chromatography technologies could offer significant time- and cost-reduction advantages.
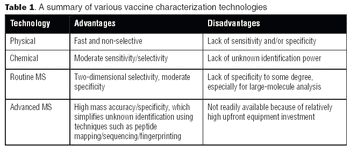
With the advent of high-resolution mass spectrometers and highly sensitive MS instruments, vaccine characterization has entered a new phase.

In animal studies, we have demonstrated that the dose of an injected H5N1 vaccine candidate can be significantly reduced by using a skin patch containing E. coli heat-labile enterotoxin (LT) applied over the injection site. LT-activated epidermal Langerhans cells migrate to the nearby draining lymph node and enhance the immune response to the injected antigen. A dry patch formulation has been optimized as a dose sparing strategy for pandemic flu and other vaccines. Iomai Corporation has developed a proprietary stabilizing formulation for the patch that allows use and storage at ambient temperature. The patch withstands temperature extremes during shipment, and is suitable for stockpiling.
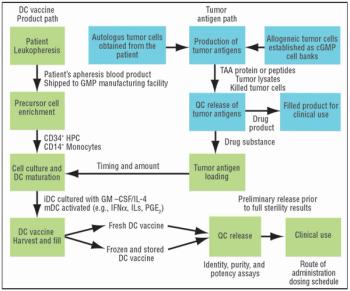
For many cell-based vaccines, the precursor monocytes or CD34+ cells are cultured with cytokines to obtain dendritic cells, which are very potent antigen-presenting cells (APCs).

The recent growth in the vaccine market has led to renewed interest in using adherent human cell lines for vaccine production. Traditionally, small-scale adherent cell line production has been carried out in roller bottles or T-flasks. Over the past few years, however, a number of companies have found multi-tray disposable bioreactors an effective method for producing high-quality drug products using adherent cells. These disposable, expandable systems have also facilitated scale up from laboratory to clinical-scale.

Vaccines against strains originating from avian flu may achieve poor yields in egg-based systems. Consequently, both public and private interest in alternative systems is high.

Any endpoint considered appropriate to support approval, whether a surrogate or a clinical endpoint, must be supported by substantial evidence of effectiveness.
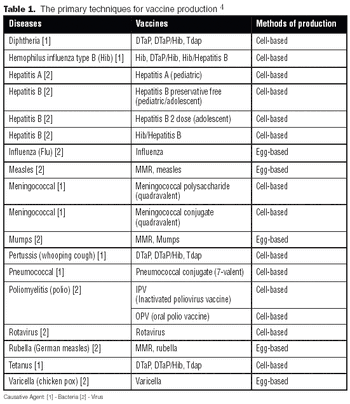
Like the egg-based vaccine production process, producing a vaccine under cGMP conditions using mammalian cells can be a lengthy process, taking a minimum of six to 12 months.
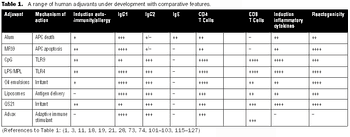
Adjuvant-caused vaccine reactions are one of the most important barriers to better acceptance of routine prophylactic vaccination.

The mounting threats of pandemic influenza, bioterrorism, and emerging infectious diseases continue to be the focus of research programs and funding initiatives, not only within governmental agencies, but also universities, private research firms, and commercial manufacturing entities. With all of these efforts, however, the question of manufacturing capacity and the ability to respond to pandemic and emerging threats continues to be a major concern.

The US Food and Drug Administration (FDA, Rockville, MD, www.fda.gov) has issued final recommendations for increasing the supply of safe and effective influenza vaccines for both seasonal and pandemic use.

Fortunately for all of us, not everyone's head is in the sand.

The FDA's (Rockville, MD, www.fda.gov) first approval in the United States of a vaccine for humans against the H5N1 influenza virus marks an important step forward in protecting the public against a pandemic influenza outbreak.
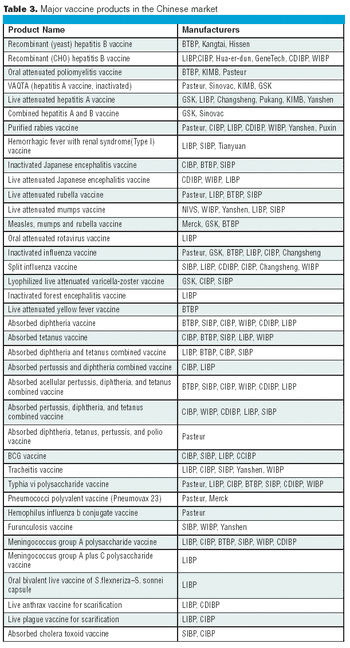
The Chinese vaccine market competition is now transferring from the former price-competition model to a technology- competition model.
Frequently asked questions on implementing and using single-use technologies