
The guidance discusses the implementation of CMC postapproval changes through a comparability protocol.

The guidance discusses the implementation of CMC postapproval changes through a comparability protocol.

FDA explains the rewards that may be associated with switching from batch to continuous manufacturing.

Jack Lew, Obama’s secretary of the treasury, announced on April 4, 2016, that the US Department of the Treasury and the Internal Revenue Service (IRS) is issuing temporary and proposed regulations to limit the “benefits of and limit the number of corporate tax inversions.” The government bodies also plan to address earnings stripping in these inversions, so it will examine past inversion deals that have already been completed.

The draft guidance outlines ways applicants can test for abuse deterrence in solid oral opioid drugs.

The agency now allows production of water for injection by non-distillation technologies.

Data integrity is a widespread, global problem that must be addressed.

The agency published guidance on immunogenicity-related considerations for low molecular weight heparin.

The directorate is looking for experts to join the European Pharmacopoeia network.

The agency is continuing CDER’s Regulatory Project Management Site Tours and Regulatory Interaction Program.

The European Directorate for the Quality of Medicines & Healthcare announces the publication of a chemometric methods chapter in the European Pharmacopoeia.

FDA’s Center for Drug Evaluation and Research publishes a list of upcoming new and revised guidance documents for 2016.

FDA issued a warning letter to Chan Yat Hing Medicine Factory for CGMP violations.

The European Pharmacopoeia rewrites in its general chapter on Raman spectroscopy.

Three associated centers in California, New York, and Florida are cited for not following proper sterilization and validation procedures and for not having the appropriate licenses to manufacture a biological drug product.

Traceability and transparency will remain elusive if manufacturers continue to approach serialization projects on a case-by-case basis.
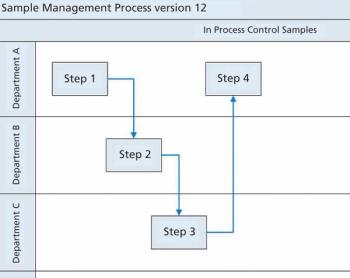
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

The United States Pharmacopeial Convention announced the recipients of the 2015–2016 Global Fellowship Awards.

Finalizing GMP requirements and quality standards for the development, manufacture, and clinical testing of ATMPs in the EU is proving to be a complex task.

FDA seeks feedback on possible analytical standards and approaches to optimize regulation of next-generation sequencing (NGS)-based in vitro diagnostic tests.
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to ensure archive records can be retrieved.
The authors discuss performing investigations of biological products.

USP responds to FDA's draft guidance on the naming of biological products.

Despite considerable investment by biotech manufacturers in developing competitive biologics for the US market, gaining FDA approval of these products has turned out to be a slow and complex process.

The new committee will attempt to recruit regulatory professionals outside of existing ICH members to improve overall global pharmaceutical regulatory harmonization.