
The report details OPQ’s accomplishments over the past five years.

The report details OPQ’s accomplishments over the past five years.

FDA published draft guidance for applicants seeking licensure of a proposed biosimilar or proposed interchangeable biosimilar.

The two agencies are collaborating to support a robust biologics marketplace by taking steps to deter anti-competitive business practices.

The FDA Commissioner plans to address drug prices, the drug approval process, and supply chain issues during his time as commissioner of FDA.

Data management is crucial in bio/pharmaceutical laboratory settings from discovery steps through clinical studies and varies based on the development phase.

Increased reliance on foreign producers raises concerns and spurs collaborations.

ICH will be taking industry comments under consideration when it revises its Q9 guideline in order to clarify QRM requirements, says Susan J. Schniepp, executive vice-president of post-approval pharma and distinguished fellow, Regulatory Compliance Associates.

The new 1800-m2 building houses more than 50 employees and features non-GMP laboratory space and a GMP analytical laboratory.

The agency has published seven guidance documents directed at the development and manufacture of gene therapies.

The FDA guidance provides an explanation of changes to user fees under the Biosimilar User Fee Amendments of 2017 under Title IV of the FDA Reauthorization Act of 2017.

The drug treats adult patients with secondary progressive multiple sclerosis with active disease evidenced by relapses or imaging features of inflammatory activity.

The facility is now equipped to handle commercial manufacturing of a sterile injectable product in a pre-filled syringe presentation.

FDA sent a warning letter to Health Pharma USA after an inspection found the company’s quality unit was not properly overseeing its drug manufacturing operations.

The agency sent a warning letter to Henan Kangdi Medical Devices Co. Ltd after an inspection found CGMP violations that included a variety of failures of the company’s quality unit.

FDA sent a warning letter to GPT Pharmaceuticals Pvt. Ltd. after inspectors found CGMP violations that included equipment that was not properly maintained.

Pressures on FDA will affect industry’s success in bringing new therapies to market.

FDA sent a warning letter to Dercher Enterprises, Inc., DBA Gordon Laboratories, for CGMP violations and adulterated drug products.

The revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world are described.

This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An understanding of global and national pharmacopoeias is crucial to understanding change processes and access to different markets.
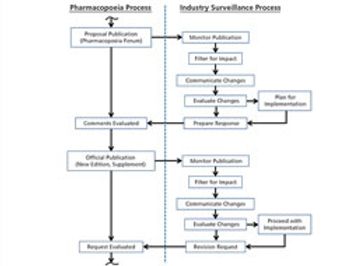
The process used to monitor and participate in pharmacopoeial changes is described.

Warning letters tell the tale of missteps by drug companies and offer a path to compliance for quality teams that monitor these enforcement actions.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.