
User fees aim to speed approvals and support inspections.

User fees aim to speed approvals and support inspections.

USP Hosts Symposium on Science and Standards

Manufacturers and regulators struggle to control phony versions of crucial medicines.

Import controls and risk strategies aim to promote quality and spur new drug development.

Multiple initiatives are moving forward to maintain US leadership in biopharm R&D.

In a special anniversary interview, Washington Editor Jill Wechsler speaks with with FDA Deputy Commissioner Deborah Autor about where the agency is headed.

Manufacturers seek clear path to develop safe-use approaches for more risky OTC therapies.

Soaring opioid use creates challenges for new drug development and supply-chain control.

Social media use raises questions about applying old standards to new information technology.

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

Contract organizations must have highly organized teams and plans to accommodate today's audits.

Pressure to approve new user fees opens the door to action on drug shortages, prices, and regulation.
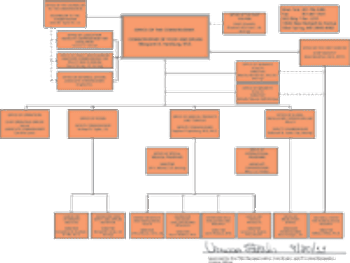
Added responsibilities and outside concerns prompt overhaul of agency's structure.

Clamor mounts over compromised care and rising costs due to lack of crucial therapies.
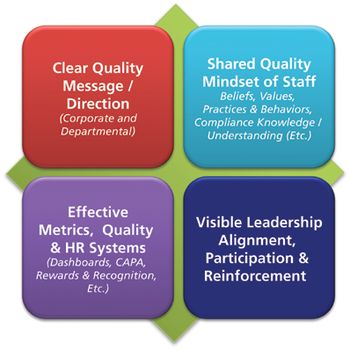
In a culture of quality, it is important that employees adopt this mindset, not because they have to, but because they understand the importance.

Manufacturers fund research and reduce prices to tackle diseases around the world.

PDUFA renewal legislation sets stage for new policies affecting revenue, research, and oversight.

Why SOPs are rarely followed, often cited, and in great need of follow-through.

US Pharmacopeia promotes horizontal standards and a product-class approach for quality attributes.

Rising imports, overseas production spur collaboration and realignment of enforcement activities.

Incorporating regulatory requirements into the product life cycle is crucial.

Follow-on versions of complex biologics require extensive expertise.

Industry struggles to curb drug abuse, diversion, and disruptions in supply to ensure access to quality products.

A rigorous cost-benefit assessment can help to chart a cost-effective path forward.

FDA, NIH, and industry seek new strategies to spur drug development and promote access to therapies.