
A Q&A with Rick Hancock, president of Althea Technologies. This article contains bonus online material.

A Q&A with Rick Hancock, president of Althea Technologies. This article contains bonus online material.
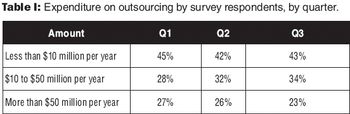
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.
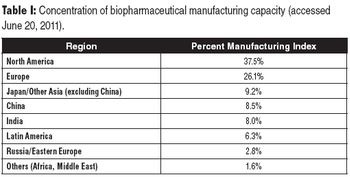
Insights from real-time ranking of global biomanufacturing capabilities.

Incorporating regulatory requirements into the product life cycle is crucial.

Knowing where key biomanufacturing facilities are located around the world is essential for decision-making.
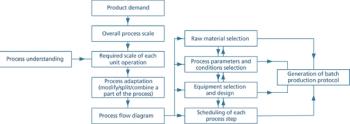
Clear documentation and open communication are essential for effective technology transfer.

Rapid microbial screening provided by contract laboratories can save companies time and money.

The authors present lessons learned from a case study of the transfer of a cell culture biotherapeutic process to a CMO.

Collaborations between Western and Indian companies may provide the best path for offshoring successfully and for developing India's readiness.

A new analysis highlights growth opportunities and challenges for contract development and manufacturing organizations.

An enterprise-wide quality management initiative is required to maintain supplier quality without sacrificing bottom-line objectives.

Lonza's bid for Patheon makes the contract manufacturing industry re-examine the one-stop manufacturer model.
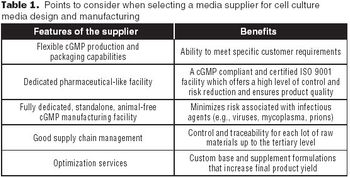
To select the right partner for media design and optimization services, several key factors must be considered.

How to ensure smooth technology transfers.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.

If Indian biologics manufacturers can establish a track record for recombinant products, enhance quality image, maintain cost competitiveness, and demonstrate technology transfer and regulatory knowhow, they are likely to be in the middle of the next boom in biologics manufacturing.
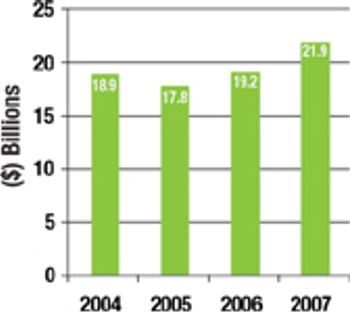
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.

There are several considerations to keep in mind when auditing an outsourcing provider.
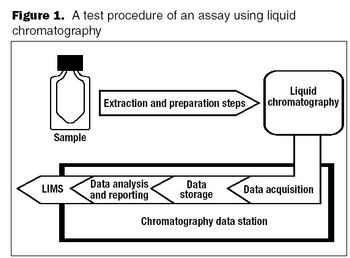
The outsourced service provider should be considered an extension of your own laboratory.

After a strategic evaluation of what activities to outsource, sponsor companies should follow key guidelines for selecting and auditing a provider and preparing quality agreements.
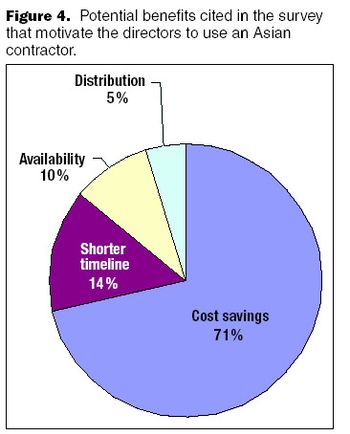
When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.

Cobra Biomanufacturing is an international full-service manufacturer of biopharmaceuticals, dedicated to designing robust processes that deliver biopharmaceutical products to its life sciences customers for preclinical through Phase 3 studies.

Discovery Laboratories (Warrington, PA) could see the end of its struggle to launch its Surfaxin (lucinactant) drug on the US market soon, as the manufacturing issues it has faced have been resolved.

The US Food and Drug Administration (FDA, Rockville, MD, www.fda.gov) issued a revised draft guidance on July 20 to help ensure that the safety, purity, and potency of biologics products is not compromised as a result of innovative, flexible manufacturing arrangements.
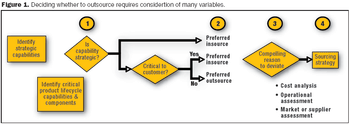
Companies need to avoid operating in a manner that is inconsistent with the priorities established in the strategic sourcing decision.