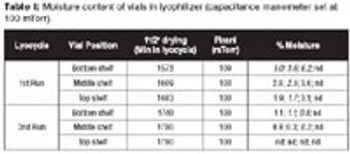
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.

Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.
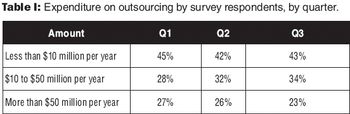
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.

Vetter held a groundbreaking ceremony for its new facility in Ravensburg.
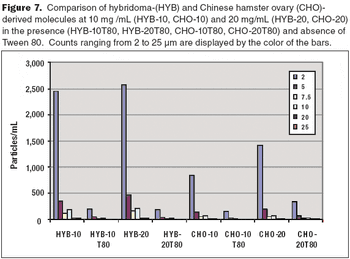
How to maintain product stability and prevent particulates.
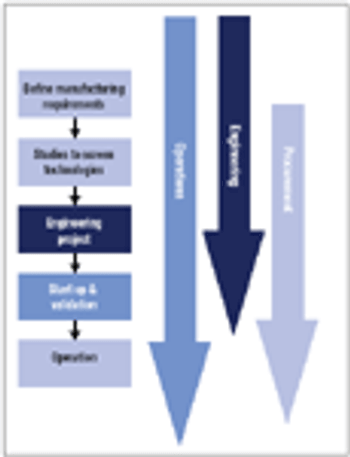
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.
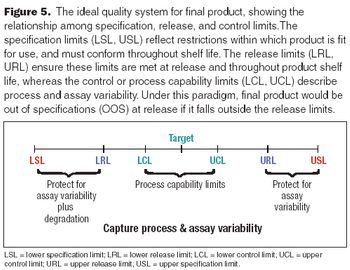
Set limits to provide incentives for process improvements.
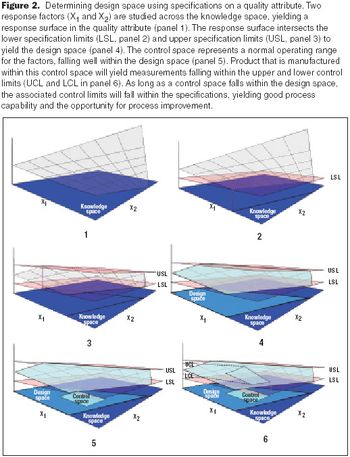
Resolve confusion about measurements.