
The new guidelines will address bioanalytical method validation and biopharmaceutics classification system-based biowaivers.

The new guidelines will address bioanalytical method validation and biopharmaceutics classification system-based biowaivers.

The company announced plans to begin shipping Inflectra to the US market in late November 2016.

A €15-million investment will be made over 2014-2016 in a biotech facility located in Tres Cantos, Spain

The agency awarded 21 new clinical trial research grants to boost the development of treatments for rare diseases.

FDA is working with EMA to treat rare diseases and keep patients involved in healthcare.

Recipharm announced that it has invested 5 million SEK in a new GLP bioanalysis laboratory in Uppsala.

The companies will collaborate the manufacture of personalized cancer vaccines.

Wallace Cameron International was cited for not registering its Wishaw, Scotland facility with FDA.

Laboratoire Sintyl S.A. received an FDA warning letter for CGMP quality violations.

A new study proposes a new way to potentially treat congenital diseases in utero.

HHS entered into a contract with BioProtection Systems Corp. for commercial manufacturing tests of an Ebola vaccine.

Marken announced the opening of a new depot and logistics operations center in Argentina.

The companies entered into a collaboration and license agreement for Crescendo’s Humabody-based therapeutics.

WuXi AppTec’s new biomanufacturing facility is its third facility in the Philadelphia, PA Navy Yard.

Results from the Phase III POLLUX trial with Janssen’s Darzalex showed that the drug was effective at reducing disease progression in patients with relapsed or refractory multiple myeloma.

The companies are collaborating on the commercialization of two biosimilar candidates in the US and Canada.

A report from the European Union Intellectual Property Office shows that the EU loses approximately €10.2 billion a year due to counterfeit medicines.

The time and resources required to finalize post-approval changes may be preventing manufacturers from modernizing facilities, or even scouting for new technology.

The company announced that it will build a new manufacturing facility at the GMR Aerospace Park at the Rajiv Gandhi International Airport in India.

Constantia Flexibles acquired a flexible packaging business from Lamp San Prospero SPA.

Value assessment initiatives are expected to have a major impact on drug use and reimbursement.

Xellia added laboratory space and personnel in Zagreb, Croatia to work on anti-infective products that combat the antimicrobial resistance problem.
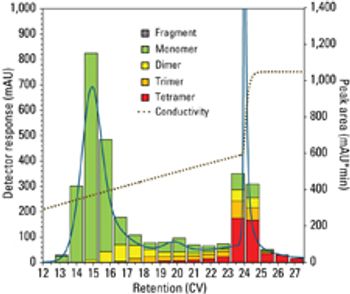
Tosoh Bioscience adds TOYOPEARL Sulfate-650F, a strong cation exchange resin that exhibits high salt tolerance, to its process media product line.

A PESU membrane is now available for Sartorius Stedim Biotech Sartocon benchtop and production-scale filtration assemblies.

Bio-Rad Laboratories’ Nuvia IMAC Resin, an immobilized metal affinity chromatography resin, is optimized for use from laboratory-scale through pilot studies to process-scale manufacturing.

Allergan entered into a licensing agreement with AstraZeneca for MEDI2070, an anti-IL-23 monoclonal antibody in phase IIB development for the treatment of patients with moderate-to-severe Crohn’s disease.

The new company will focus on R&D of cancer treatments.

The Phase III trial examined guselkumab compared with a placebo and Humira at treating moderate-to-severe plaque psoriasis.

The hospital received a five-year $5 million grant from CDC to survey for communicable diseases in children and evaluate vaccine effectiveness.