
Guide to Disposables Implementation

Guide to Disposables Implementation

A case study evaluates the performance, control of operations, productivity, and cost savings of a single-use system.
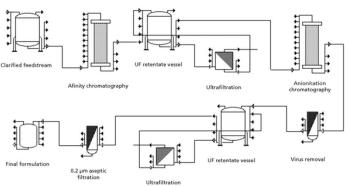
Single-use TFF offers the greatest savings in clinical and contract manufacturing, where the scale is low and changeovers are frequent.
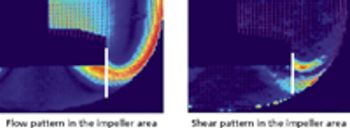
Switching to single-use bioreactors can have financial and performance benefits.

BPSA eases confusion over extractables and leachables testing through guides.
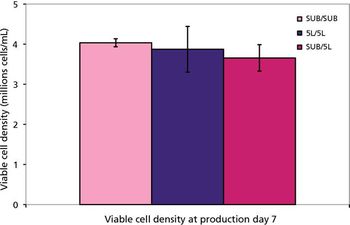
Despite different material, agitation, and aeration, the performance of the disposable bioreactor is similar to that of stainless steel bioreactors.