
The Evolution of Vaccines

The Evolution of Vaccines

Release testing involves both standard potency assays and unique assays (particle size, NA activity) developed to ensure the physical, chemical, and biological stability of this type of vaccine.

Adjuvant activity can be greatly improved by appropriate formulation of cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODNs).
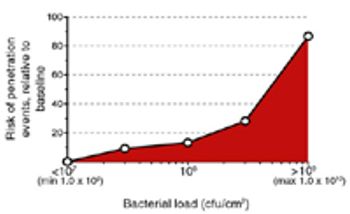
Filterability and bacterial retention must be verified very early in process development to ensure successful sterilizing filtration validation.

Adjuvant activity can be greatly improved by appropriate formulation of cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODNs).