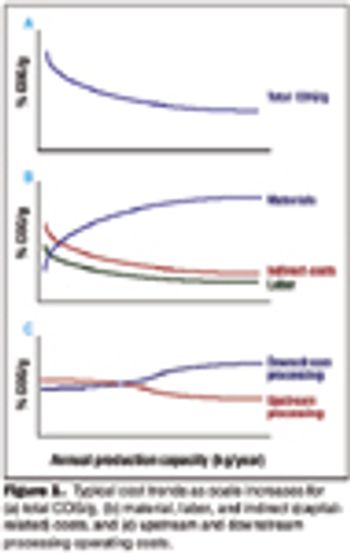
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

Altering the order of operations, using new resins, and increasing dynamic binding capacity can obviate the need for major facilty changes.

A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase therapeutic antibodies.
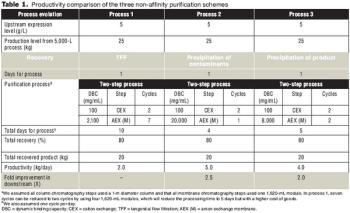
In three non-affinity purification processes based on cation exchange capture with high binding capacity, applying a host cell protein exclusion strategy enabled robust scale up and better economics.
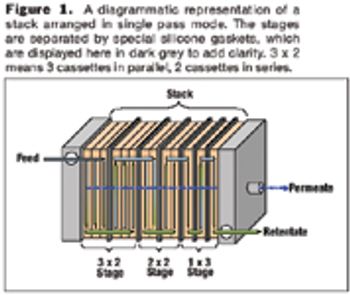
New techniques can greatly improve the MAb purification process.
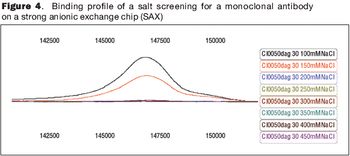
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.