
Alternative delivery methods for biologics continues to be explored that offer less invasive, less painful administration.

Alternative delivery methods for biologics continues to be explored that offer less invasive, less painful administration.
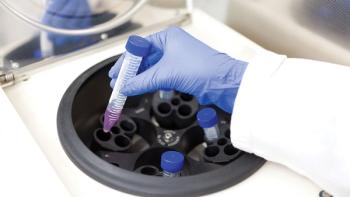
This case study describes how the risk assessment of the first-to-market single-use disc-stack centrifuge was conducted.
Weighing development costs/resources and performance benefits is essential.
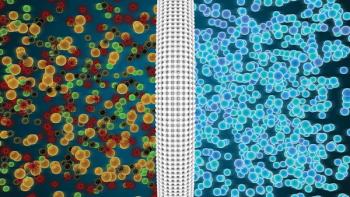
Collaboration between technology providers and biopharma manufacturers are enabling both evolutionary and novel developments.
Platform processes and effective risk assessments help overcome time and cost challenges.

Viral vectors and other complex biologic modalities require more specificity and higher sensitivity to detect and distinguish contaminants.

As the field of bioanalytical testing evolves, it is important for drug developers to stay at the forefront of the advancements to ensure they remain competitive.

A coordinated and international response is needed to help control the latest mpox outbreak in Africa.

Updating your audits and inspections program ensures inspection preparedness, says Siegfried Schmitt, vice president, Technical at Parexel.