
A holistic approach to validation and quality assurance is essential.

A holistic approach to validation and quality assurance is essential.
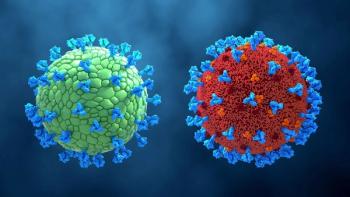
Analytical and functional characterization of virus-like particles enables process reproducibility and product consistency.

Establishing an automated inline dilution system can potentially ease bottlenecking delays resulting from higher upstream yields.

Viral clearance processes and guidance must evolve along with newer biotherapeutic modalities.

The industry is moving beyond cleaning’s “low tech” image to embrace science-based limits and statistical approaches to control.

New advancements in lab data management technologies include devices with a fully integrated SDMS, a cloud-based and an online ELN, an ELN featuring a virtual assistant, and updated LIMS software.

Scaling needs for potential COVID-19 vaccines depend not only on capacity, but also on supply chain challenges and technological hurdles.

Manufacturers and regulators accelerate R&D and production of new vaccines and therapies.

After a difficult year, biopharma science delivers promising results.

Virtual audits, virtual training, and more robust quality agreements may become positive impacts on the industry, says Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates.