
A Single-Use System for Safe Freeze–Thaw

A Single-Use System for Safe Freeze–Thaw

To achieve manufacturing excellence, biopharma companies must adopt four key operating principles.
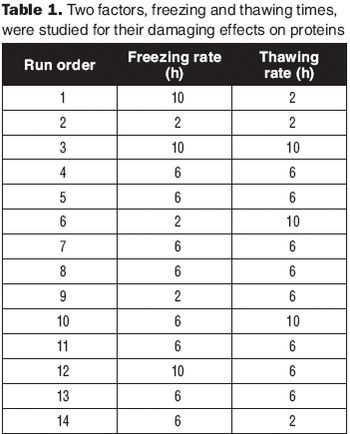
Apply a DoE strategy to test several formulations in parallel.

Covance's deal with Sanofi-Aventis demonstrates the power of scale and scope.

Biotech, and bioprocessing more specifically, is a great place to be. But it's time for a new adventure.

Managing partnerships for the greater good.

Changes on Capital Hill create uncertainty for healthcare reform, drug regulation, and biomedical research.

Evaluate and communicate risk to stakeholders.
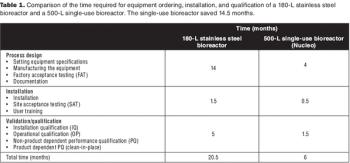
Sanofi Pasteur's disposables implementation plan is part of a larger evaluation of technology innovation. Here's how they approach it.