
To expand coverage amidst the economic crisis, Obama will be looking hard for ways to cut healthcare costs.

To expand coverage amidst the economic crisis, Obama will be looking hard for ways to cut healthcare costs.
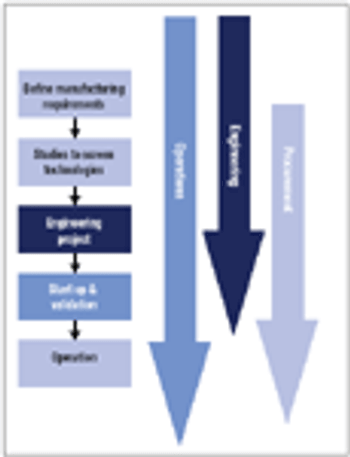
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.
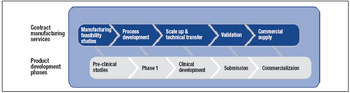
Four reasons why outsourcing may be the best option, and key factors to consider when selecting a provider.

To stave off quality issues associated with production processes, a centralized and enterprise-wide quality management initiative must be enforced.

A clear oversight framework for biomarker clinical laboratory tests used in personalized medicine is required.

No time for QbD? How to convince management to make it a priority.

A closer look at the past year's development in India.

The meltdown in the financial markets represents a sea change in the world of financing that will continue to affect the flow of much needed capital into the sector for the foreseeable future.

The FDA's revised process validation guidance manages to explain the underlying concepts of Quality by Design without every using the phrase.
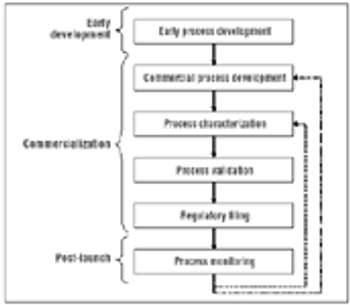
Using multivariate experiments to define acceptable ranges.
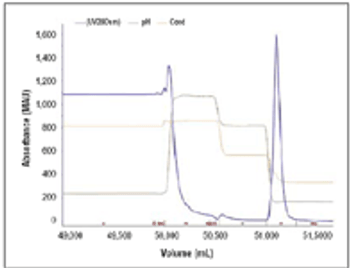
Results from a process developed for a commercial antibody.