
The complex world of international regulations, standards, and guidelines on sterile manufacturing of parenteral products is deciphered and reduced to a set of matrix tables.

The complex world of international regulations, standards, and guidelines on sterile manufacturing of parenteral products is deciphered and reduced to a set of matrix tables.

Biopharmaceuticals are perceived as being at risk of transmitting spongiform encephalopathies to patients, although there has never been such an incident. Clearance studies such as the one described in this article (using a TSE model) can validate inactivation and enhance confidence in the safety of therapeutics produced using animal-derived cell culture supplements.

FDA and manufacturers seek to curb bogus drugs, while legislators consider liberalizing import policies to cut pharmaceutical costs.

Vaccine process development is complex, and so are the documents required before clinical trials begin. Technical writers and editors can effectively coordinate the timely authoring, reviewing, and auditing of regulatory documents, minimizing filing delays.

The medical benefits of transgenes - effective against diseases such as cancer, HIV, malaria, and tuberculosis - require either a viral or nonviral vector. Nonviral vectors have lower immunogenicity, better safety profiles, and improved stability, as well as being less expensive and easier to produce. The first article in this series reviewed techniques suitable for the purification of plasmid DNA in the absence of added RNase. This article investigates a downstream process capable of producing gram quantities of pure plasmid DNA.

The author shares a case example that applies an eight-step structured approach to the front-end engineering of a vaccine R&D fill-and-finish facility project. Assumptions, issues, action items, owner interface, and interdisciplinary coordination are covered to meet the challenges of timing, technology, compliance, and cost.
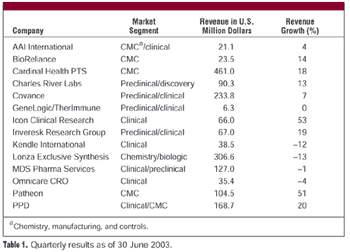
CROs and CMOs have reported on their second quarter financial performance during July and August. Although revenues and profits were up for most companies reporting, the discussions of market conditions surrounding the financial results had a somewhat unsettled feel. Most contractors expect robust revenues in the second half of 2003 but are clearly nervous about whether the activity will actually materialize.