The authors outline cell-line development and process scale-up for an antibody program in which the antibody requires additional processing by a site-specific enzyme for correct functionality.

The authors outline cell-line development and process scale-up for an antibody program in which the antibody requires additional processing by a site-specific enzyme for correct functionality.

Quality, flexibility, and cost savings are driving use of perfusion technology in biosimilars manufacturing.

Multiple methods are required for detecting and removing protein impurities.

Congressional partisanship creates noise, but no funding for Zika virus research.

A step-wise process is used to characterize glycans and understand the functioning of a molecule for biosimilar development.

Regulators and manufacturers address economic and ethical issues for scarce medicines.
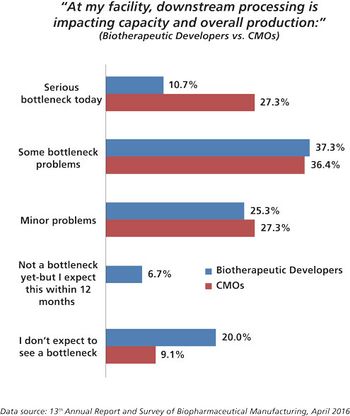
CMOs are working hard to improve performance by investigating new technologies for filtration and purification.

Susan Schniepp, Distinguished Fellow at Regulatory Compliance Associates, discusses the regulatory requirements for improving manufacturing lines.

Industry experts discuss the challenges of using single-use systems in biopharma manufacturing.
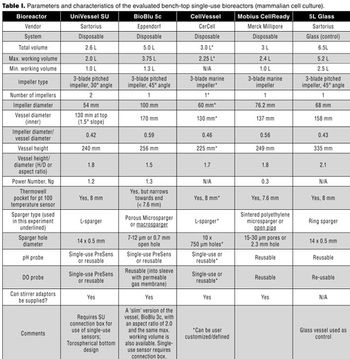
The authors present a case study in which four single-use vessels were fitted to an existing bioreactor system.
The study demonstrates a systematic approach to stabilize PBS-formulated mAbs against freeze-thaw degradation.

The author outlines key functional attributes to assess a small-scale model.

The Titan Syringe Pump from Syrris is a continuous flow chemical processing module suitable for lab-, pilot-, and production-scale applications.

The IsoBag from MilliporeSigma is used for the convenient transfer of contact and settle plates to production isolators.

Click the title above to open the BioPharm International August 2016 issue in an interactive PDF format.