
Advances in Protein Analysis

Advances in Protein Analysis

There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.

A one-day sign off for batch records is considered a best practice in the industry.

After a series of government reforms, the Japanese pharma market is making a comeback.

Recently developed immunoassay technology platforms reduce sample volume requirements and improve cycle times.

Import controls and risk strategies aim to promote quality and spur new drug development.

A look back at the history of orphan drugs in the industry.

A team from Northwestern University has demonstrated the feasibility of topical delivery of small interfering RNA (siRNA).
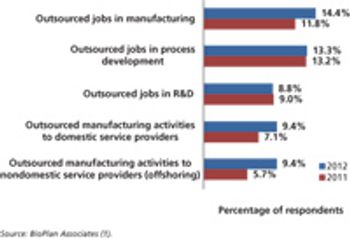
Budgets for biopharma activities are gaining in select functional areas, except outsourcing.
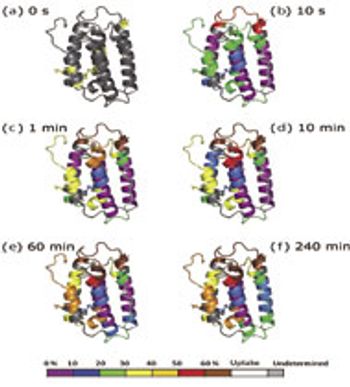
In HDX studies, data are produced across multiple time points, multiple species, and with replicates.
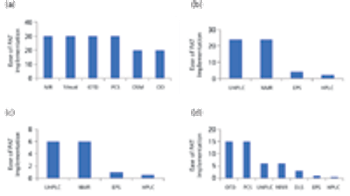
The authors review the various analytical methods that can enable use of PAT.
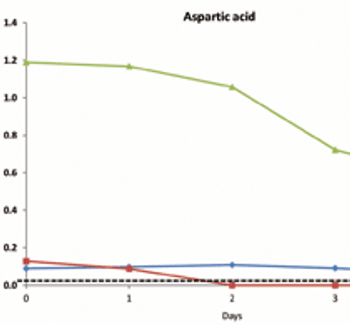
Current expectations in bioprocessing and a framework for using NMR to enhance a QbD approach.

Now that the Supreme Court has upheld the Affordable Care Act, what's next for biopharma?
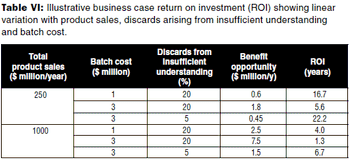
The author describes a methodology for developing a per product qualitative and semi-qualitative business case for applying QbD to a biopharmaceutical product.
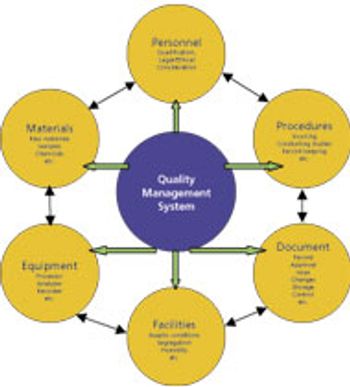
Members from an ASQ working group provide analytical methods to enable PAT.