
Top 1000 Biomanufacturing Facilities

Top 1000 Biomanufacturing Facilities
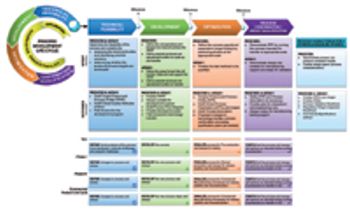
Achieving multiproduct development within shortened timelines.

China rises to the top as a destination for international outsourcing.

The author looks at strategies to minimize particle levels in the finished product when using single-use technologies downstream of final capabilities.

US Pharmacopeia promotes horizontal standards and a product-class approach for quality attributes.

Rising imports, overseas production spur collaboration and realignment of enforcement activities.
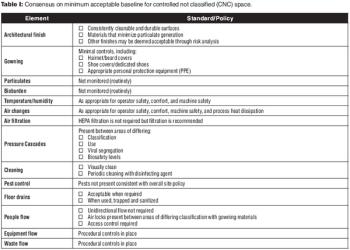
The authors re-examine environmental controls in the context of technical advances in manufacturing.

Industry may be its own obstacle to success in achieving the desired high-performance state.

Are biosimilars the next big thing or just the next big bubble?
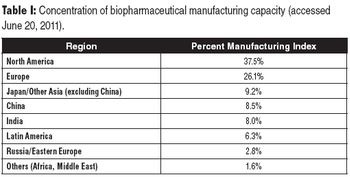
Insights from real-time ranking of global biomanufacturing capabilities.