
Facilitate Cell Culture Process Development

Facilitate Cell Culture Process Development
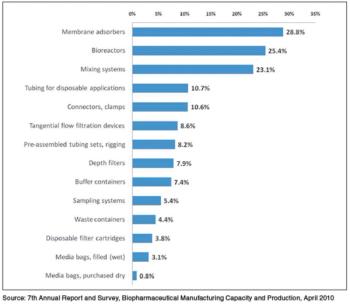
Although single-use systems are widely used in upstream unit operations, their acceptance in downstream processes has been slow.

Regulatory relief requires that regulators trust companies to know what they are doing, and to do it-consistently.

This month, we catch up on major developments around the world and their implications for the contract services industry.
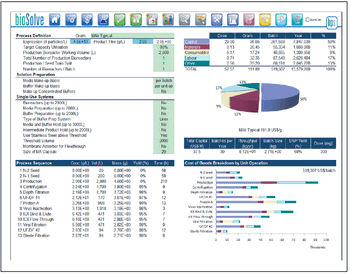
An analysis of flow rate, load density, viral clearance, and cost.
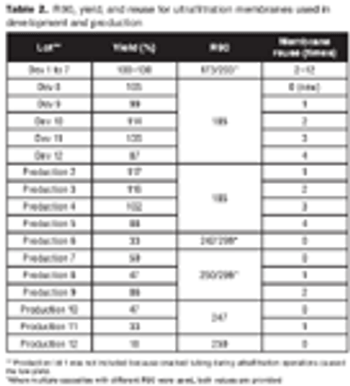
How to balance porosity, plugging, and lot-to-lot variability in filters.

In light of the new ruling, patent licensees may want to re-evaluate the strength of their licensed patents.

Plant closures, product recalls prompt FDA re-evaluation of GMP enforcement efforts.

How a Big Pharma company tackled the move to disposable bioreactors.
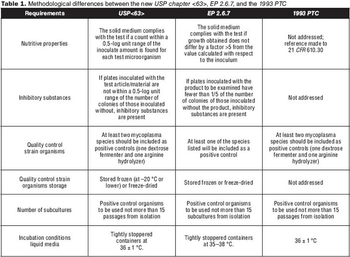
What you need to know about USP chapter <63>.
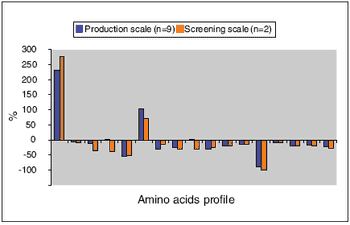
Rolling-tube system balances scale-up accuracy and thoroughput.