
An Effective Way to Ensure Consistent Protein Expression

An Effective Way to Ensure Consistent Protein Expression

A culture of quality that emphasizes business objectives, risk management, and the informed application of technology can improve compliance.
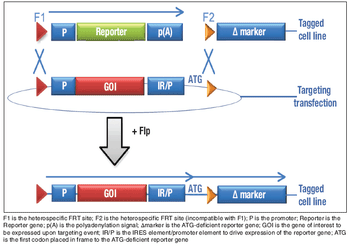
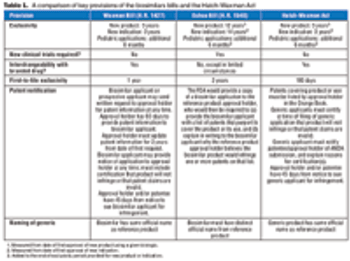
The introduction of two rival bills has intensified the long-simmering debate on biosimilars regulation in the US.

Although the markets closed on a positive note in May, it will still take many more months before biotech starts on the road to full recovery.

The truth is, we should have been afraid of H1N1, because the threat of a flu pandemic is real.

By following key strategies, companies can reduce the risk and increase the benefits of outsourcing analytical development and testing
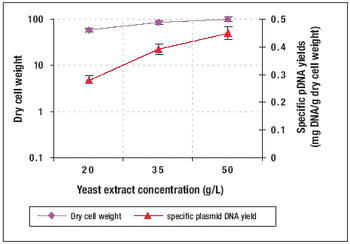
How to produce Plasmid DNA in a high-cell-density culture.

Biopharmaceutical companies must follow an active approach to managing their supply chains.

The FDA seeks new strategies for improving the safe use of opioids and other high-risk medicines, including erythropoiesis stimulating agents.