
Optimize Processes by Using CoGs Modeling

Optimize Processes by Using CoGs Modeling

International outsourcing and rising theft spur regulatory action and manufacturer oversight.
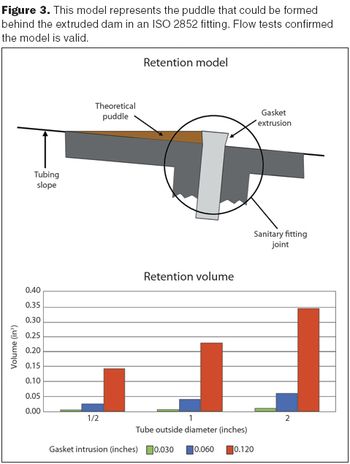
How to control compressive loads on seal materials.

The new US legislation will forever alter the commercial landscape for biologics.
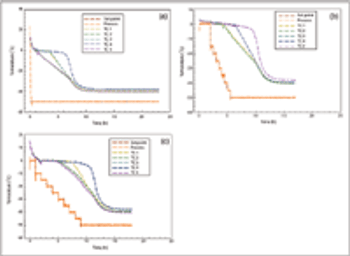

Being open to unforseen events may avert risk or create opportunities.

New R&D models must be tried, but it will take time to see if they work. In the meantime, a new kind of threat is on the horizon: biobetters.
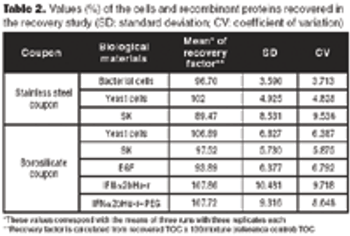
Case studies show TOC is effective for cleaning validation.
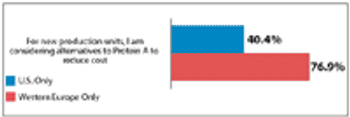
Biomanufacturers and vendors are now exploring more cost-effective purification technologies.
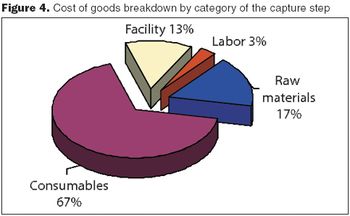
Combine cost analyses with QbD to improve operations and lower costs.