
Investing in BioPharma Outsourcing

Investing in BioPharma Outsourcing

Stephen Taylor PhD, vice-president and commercial director at Fujifilm Diosynth Biotechnologies, addresses some of the challenges facing biologics outsourcing.
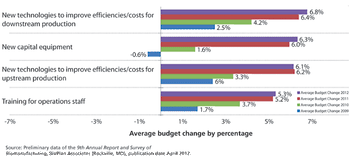
Industry optimism is on the rise for 2012.

China's healthcare reforms generate uncertainty for its domestic pharmaceutical market.

The census reveals the state of the population of India's health and the potential for growth in the healthcare market.

New educational programs are key to the industry's future and to safe, available drugs.
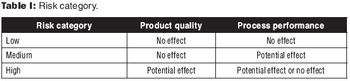
Strategies for transfer of the manufacturing process.
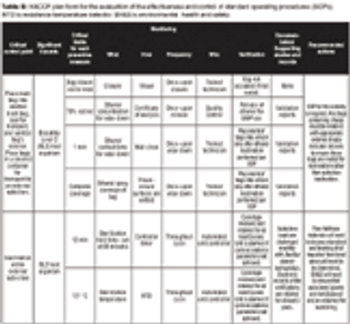
A Risk-Management Case Study.
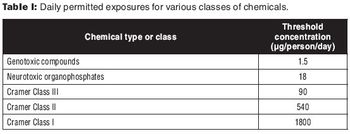
This article is the second in a two-part series on extractables and leachables.

Industry announces plans for year ahead at annual JPMorgan Global Healthcare Conference.

This article outlines methods, validation standards, and documentation of sterilization of single-use products using gamma irradiation.

This month, we revisit "Industrial-Scale Mammalian Cell Culture, Part I: Bioreactor Design Considerations."

Introducing a new way to think about sharing information in a patent-driven industry.

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

The difficulties of doing business in China are offset by its adoption of more stringent GMP standards.