
Siegfried Schmitt, vice president, Technical at Parexel, provides best practices for switching from paper-based to automated processes.
Siegfried Schmitt, PhD, is Vice President Technical at Parexel International, Siegfried.Schmitt@parexel.com.

Siegfried Schmitt, vice president, Technical at Parexel, provides best practices for switching from paper-based to automated processes.

Regulatory inspection reports can be a valuable resource for quality management, according to Siegfried Schmitt, vice president, Technical at Parexel.

System documentation should include a system description, history, validation information, and references, according to Siegfried Schmitt, Vice President, Technical at Parexel.

Auditing distribution suppliers provides understanding and documentation of the services performed.

Updating the quality technical agreement will clarify any expectations and limitations, says Siegfried Schmitt, vice president, Technical at Parexel.

Siegfried Schmitt, vice president, Technical at Parexel, offers insight into FDA’s guidance on performing operations during COVID-19.

It is important to consider the feasibility, benefits, and limitations of each type of audit in advance.

Having a better understanding about compliance will be of benefit when looking for a job or for furthering one’s career, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.
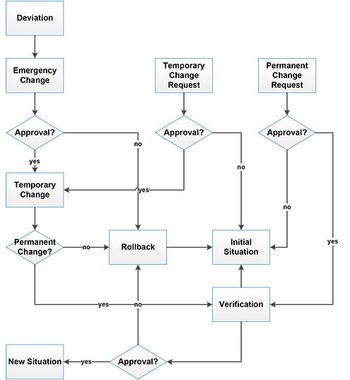
No matter why change may be needed, it is important to comply with all the relevant regulatory requirements, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

It is good industry practice to clarify the precise remit for each of the reviewers of a controlled document, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

Providing regulators with a holistic approach to addressing deficiencies is the best response to an inspection, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

Cultural and language discrepancies during an audit can be resolved using what many call a “playbook,” says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

We need to understand both sides when it comes to audits, says Siegfried Schmitt, PhD, vice-president, technical, PAREXEL Consulting.

Simplification is crucial to maintain data integrity, according to Siegfried Schmitt, PhD, vice-president, technical at PAREXEL Consulting.

Understanding the differences in inspection processes is the key to successful global expansion, according to Siegfried Schmitt, PhD, vice-president Technical, PAREXEL Consulting.

Building up relevant expertise in-house will make writing spec sheets for software easier, according to Siegfried Schmitt, principal consultant at PAREXEL.

Detailed process descriptions and robust documentation aid in compliance as well as training, says Siegfried Schmitt, principal consultant at PAREXEL.

Knowing and addressing regulatory expectations early on can avoid unexpected delays later, says Siegfried Schmitt, principal consultant at PAREXEL.
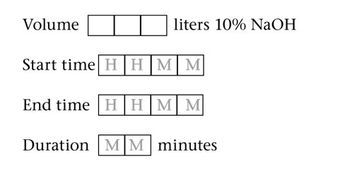
Monetary benefits will outweigh the hassle of batch record harmonization, says Siegfried Schmitt, principal consultant at PAREXEL.

In-house experts can help select the right systems and suppliers, making validation and compliance easy, says Siegfried Schmitt, principal consultant at PAREXEL.

Enterprise-wide processes, procedures, and systems are the keys to data integrity and peace of mind, according to Siegfried Schmitt, PhD, principal consultant at PAREXEL.

Siegfried Schmitt, PhD, principal consultant, PAREXEL, discusses how to prepare for an inspection by a foreign regulatory agency.

The level of formality in change control may be holding back your SOP progress, according to Siegfried Schmitt, principal consultant at PAREXEL.

The differences between the quality control and quality assurance units can be found in their names, according to Siegfried Schmitt, principal consultant at PAREXEL.

The opening presentation gives the company a chance to put their best foot forward, according to Siegfried Schmitt, principal at PAREXEL.

Siegfried Schmitt, PhD, Principal Consultant at PAREXEL, discusses how to mitigate risk in a global regulatory environment.

Siegfried Schmitt, PhD, principal consultant, PAREXEL, discusses how to handle audits and inspections during business expansion.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to report quality metrics to FDA.
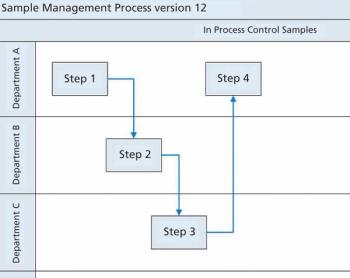
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to ensure archive records can be retrieved.