
The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.

Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology Delhi and a member of BioPharm International's Editorial Advisory Board, Tel. +91.9650770650, asrathore@biotechcmz.com.

The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.
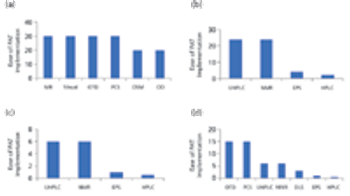
The authors review the various analytical methods that can enable use of PAT.

Challenges of vaccine development include regulatory, technical, and manufacturing hurdles in translating a vaccine candidate into a commercial product.
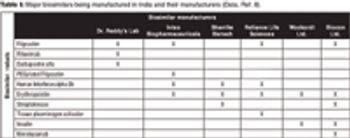
The author explains the current status of India, the challenges, and recommendations that may alleviate these challenges.
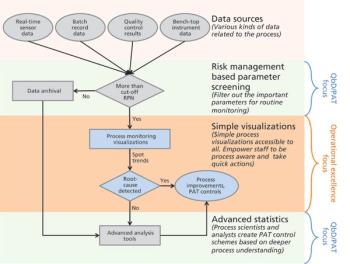
The authors focus on operational excellence in manufacturing of biotechnology therapeutic products in the QbD paradigm.
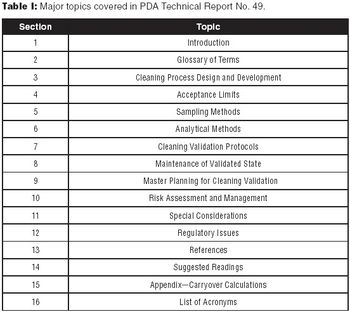
The authors encourage biotech manufacturers to consult PDA Technical Report No. 49 for a detailed perspective on current practices and issues in biotech cleaning validation.

An approach to reduce batch time, increase productivity, and decrease costs.

Evaluate and communicate risk to stakeholders.
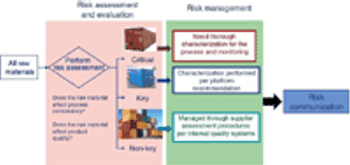
Adequate characterization of materials protects product quality.

Regulatory flexibility can make continuous improvement possible.
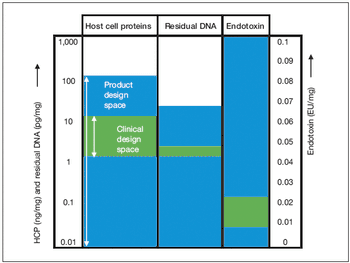
Key considerations for defining your overall control strategy.
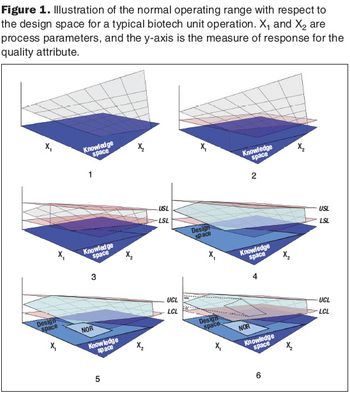
Second in a three-part series that discusses the complexities of QbD implementation in biotech development.
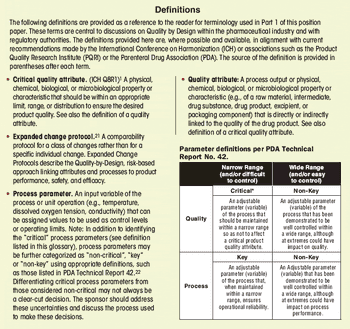
First in a three-part series that discusses the complexities of QbD implementation in biotech product development.
Select the best approach to determine critical quality attributes.
How to maintain product stability and prevent particulates.

This article reviews some of the commonly used approaches for process monitoring as well as the evolution of process monitoring in the Quality by Design (QbD) paradigm.
Choosing the right tools to enhance the process.

Understanding the relationship between the process and CQAs.
Using multivariate experiments to define acceptable ranges.
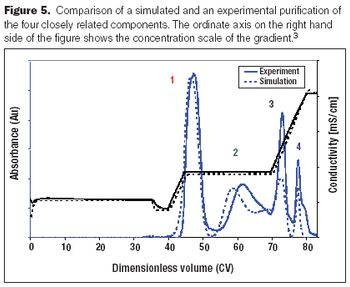
A review of some recent contributions in process chromatography.
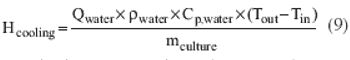
Process-modeling tools can ensure smooth tech transfer.

A case study investigated the root cause of failures in sterile filtration by evaluating the effects and interactions of four operating parameters.

Multivariate data analysis can help biotech manufacturers deepen their process understanding.


Process monitoring ensures that the process performs within the defined acceptable variability that served as the basis for the filed design space.

The number of biotechnology-based human therapeutic products in the late-stage pipeline, and the average cost to commercialize a biotech product, have steadily increased. This has required biotech companies to use economic analysis as a tool during process development and for making decisions about process design. Process development efforts now aim to create processes that are economical, as well as optimal and robust.
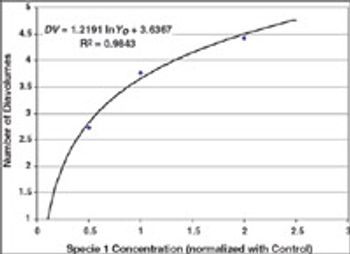
Case studies were run to test Process Analytical Technology applications for protein refolding, diafiltration, and cation exchange chromatography. It is shown that it is feasible to design control schemes that rely on measurement of product quality attributes and thereby enable real-time decisions.
Misinterpreting the effluent profiles obtained during tracer measurements performed for determining packing quality can often lead to excessively large percolation velocities and exaggeration of packing problems. Highly useful and reliable information can be obtained through characterization of tracer effluent curves using the method of moments, information that could be critical for successful scale-up of chromatographic steps. This is the sixth in the "Elements of Biopharmaceutical Production" series.

Creation and qualification of scale-down models is essential for performing several critical activities that support process validation and commercial manufacturing. This combined article is the fifth in the "Elements of Biopharmaceutical Production" series. Part 1 (March 2005) covered fermentation. In this segment, we present some guidelines and examples for scale-down of common downstream unit operations used in biotech processes - chromatography and filtration.

Lot-to-lot variations between raw materials can greatly impact process performance.