
Andrew Bulpin, head of Process Solutions, MilliporeSigma, shares insights on characterizing CQAs in biopharmaceutical development and the different tests that should be carried out when assessing an investigational drug.
Adeline Siew is science editor for BioPharm International.

Andrew Bulpin, head of Process Solutions, MilliporeSigma, shares insights on characterizing CQAs in biopharmaceutical development and the different tests that should be carried out when assessing an investigational drug.

Materials in contact with a drug must be fully characterized to ensure they do not negatively affect the safety and efficacy of the product.

Process analytical technology tools have enabled manufacturers to monitor and control their production processes.

Robust materials management, supplier quality management, quality control, and business continuity planning are essential to ensure continuous supply of single-use bags.

Advances in wearable devices have made it possible to deliver high-volume, high-viscosity biologics.

Experts share insights on the various methods used for purity and impurity analysis of therapeutic proteins.
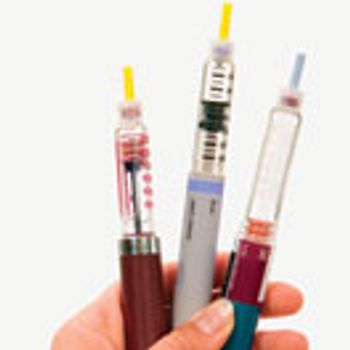
Siliconization is a key process step in the manufacturing of prefilled syringe systems.

There is a lot of interest in delivering biologics via non-invasive routes in attempt to improve patient compliance and convenience.
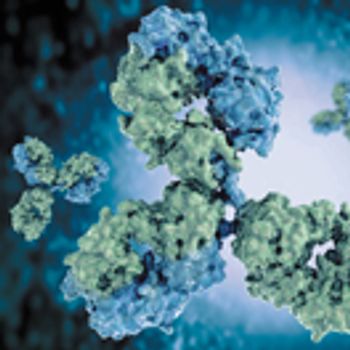
OrlaSURF technology can be used for the development of target-binding assays to monitor the binding of an ADC to its antigen.

Andrew Bulpin, head of Process Solutions Strategic Marketing & Innovation at MilliporeSigma spoke with BioPharm International about the requirements of expression systems in biosimilar development.

The baculovirus-insect cell system can produce large quantities of complex protein in a short period of time.

Interactions between biologic drug products and the components of prefilled syringes can cause protein aggregation, but there are alternative materials that can help mitigate this problem.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.
Understanding and preventing protein aggregation is crucial to ensuring product quality and patient safety.

Experts discuss the advances in single-use technology for downstream bioprocessing applications.

Experts discuss the advances in single-use technology for downstream bioprocessing applications.

Mike Jenkins, general manager of Catalent Biologics discusses the evolving landscape of the biologics market and hurdles faced in the development and manufacture of these innovative products.
Review the importance of characterization studies during biosimilars development and related analytical methods.

Scientists at A*STAR's Genome Institute of Singapore (GIS) have identified genes that could be potential targets for anticancer agents in the treatment of aggressive breast cancer.

Adaptimmune has opened a Phase I/IIa ovarian cancer trial to evaluate its genetically engineered T cell treatment, with enhanced antitumor activity.

The report highlights a need for greater third party certification to ensure GMP vigilance.

Seattle Genetics could potentially receive approximately $500 million in fees, milestones and royalties.

Amgen and Zhejiang Beta Pharma have signed an agreement to form a joint venture to commercialize Amgen's Vectibix (panitumumab) in the Chinese market. The aim is to quickly and efficiently deliver Vectibix to patients in China. Vectibix is a fully human anti-epidermal growth factor receptor (EGFR) antibody approved by FDA for the treatment of metastatic colorectal cancer.

Amgen and Zhejiang Beta Pharma have signed an agreement to form a joint venture to commercialize Amgen's Vectibix (panitumumab) in the Chinese market. The aim is to quickly and efficiently deliver Vectibix to patients in China. Vectibix is a fully human anti-epidermal growth factor receptor (EGFR) antibody approved by FDA for the treatment of metastatic colorectal cancer.

Genentech announced that FDA has approved Actemra (tocilizumab) for the treatment of polyarticular juvenile idiopathic arthritis (PJIA) in children aged two years and older.

Pharmaco-Kinesis Corporation (PKC) announced that it is developing the first nanodrug combination of Merck's temozolomide and Celgene's thalidomide (in a 50:50 ratio) for the treatment of gliomas and other cancers. PKC will collaborate with the University of California, San Diego's department of nano-engineering at the Moores cancer centre to develop the nanodroplet formulation.

Bayer announced that it has signed a merger agreement with US-based birth-control specialist Conceptus.

BioOutsource, a contract testing services provider to the biopharmaceutical industry, has opened a new facility in Cambridge, Massachusetts. The facility marks the company's first North American offices, which were opened in response to increasing demand for the company's bioanalytical and biosafety services, particularly in the field of biologic and biosimilar characterisation.

AstraZeneca and BIND Therapeutics have formed a strategic collaboration to develop and commercialise BIND's Accurin, a targeted and programmable cancer nanomedicine, based on a molecularly targeted kinase inhibitor developed and owned by AstraZeneca.

Regeneron, a biopharmaceutical company based in New York, has announced plans to expand its corporate headquarters and laboratories in Westchester. This expansion will create more than 400 new highly skilled jobs and further cement Hudson Valley's reputation as an emerging epicenter for biopharmaceutical growth.

Published: February 18th 2013 | Updated:

Published: January 7th 2013 | Updated:

Published: February 25th 2013 | Updated:

Published: February 26th 2013 | Updated:

Published: January 8th 2013 | Updated:

Published: March 1st 2014 | Updated: