
QbD in Practice–Part 2: The Regulatory Filing

QbD in Practice–Part 2: The Regulatory Filing

A new analysis highlights growth opportunities and challenges for contract development and manufacturing organizations.

Suppliers, manufacturers, and governments must work together to plan how best to develop and deploy disposable systems for emergency response.
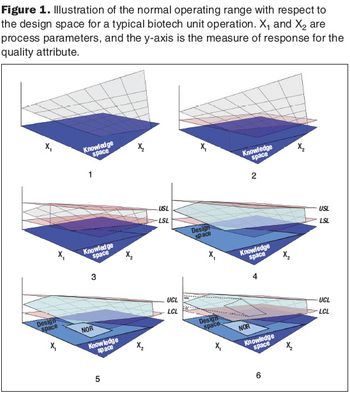
Second in a three-part series that discusses the complexities of QbD implementation in biotech development.

Gaining a license can be a complex process, but a few key tips can help you avoid common pitfalls and patent infringements.

Vaccine research and development is surging, but continues to face manufacturing and regulatory challenges.

Do we really have to choose between saving money and saving time?
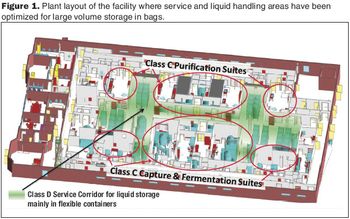
A case study compares capital costs, operating expenses, and NPV for a new MAb plant.

A case study assesses freezing time and physical robustness under stress.

Drug counterfeiting has become a major problem for the FDA today; a variety of solutions is needed.