
Making Chemically Defined Media Work

Making Chemically Defined Media Work

Best practices to strengthen supplier quality management.

The pathway for biosimilar approval in the US has been set. But are US patients too far behind Europe?
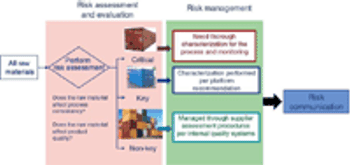
Adequate characterization of materials protects product quality.
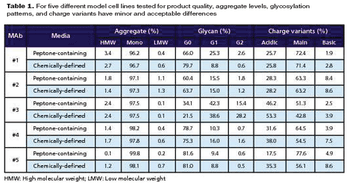

These new analytical methods can reduce analysis and product development time.

Comparative effectiveness poses challenges for drug manufacturers.

China's regulators acknowledge the quality gap, and have a plan for steady improvement.