
We often assume we know what success looks like for our partner, but we never ask them, or take the time to write it down.

We often assume we know what success looks like for our partner, but we never ask them, or take the time to write it down.

The first part of this article, published in the September 2006 issue, discussed general strategies for validation extensions to other test method components, laboratories and even different test methods.1This second part provides practical tips on how to maintain test method suitability long after the formal completion of analytical method validation (AMV) studies.
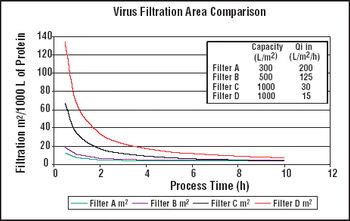
It is important to ensure that flow decay during processing is comparable to that observed during retention studies.
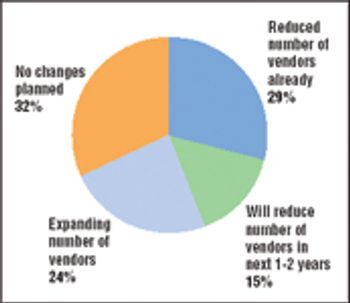
A major shift is occurring in the way the biopharmaceutical industry sources goods and services. Price pressures at the retail end of the value chain and a difficult fundraising environment are forcing biopharmaceutical companies to take greater control of their costs. Purchased goods and services, including contract research and manufacturing services as well as raw materials and laboratory supplies, are a major expense in most companies, so control of those purchasing decisions is coming in for special scrutiny.

Formulations for pulmonary inhalation comprise spherical, porous particles that are 1–3 microns in diameter.

Fear leads to bad decisions on a Wall Street trading floor, or at a high-stakes Vegas poker table.