
Combine Mechanistic Modeling and DoE to Streamline QbD

Combine Mechanistic Modeling and DoE to Streamline QbD

Too many REMS cause headaches for doctors and the industry.
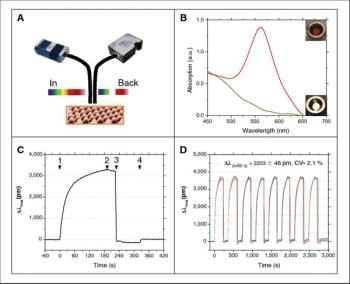
Use it label-free, or add labels to detect contaminants in solution.

What small biotechs need to know about quality management systems.

An in-depth analysis of the patent provisions of the new legislation.

The third holy grail of biosimilars: interchangeability.

How current economic conditions affect your build-or-buy decision.
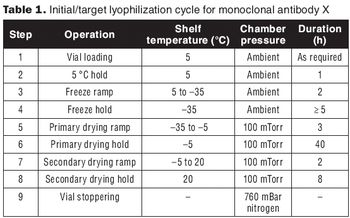
Develop a relevant design space without full factorial DoE.