
A Better Way to Study Leachables from Container Closures

A Better Way to Study Leachables from Container Closures

Given today's challenges, traditional strategies were bound to change. Now, there is one more big threat (or opportunity?) on the horizon.

The ability to deal with the complexity of the clinical supply process has shifted the balance of power to clinical supply chain specialists.

As the capital markets in the US and globally continue to strengthen, the biotech industry can expect to see a rise in initial public offerings this year.
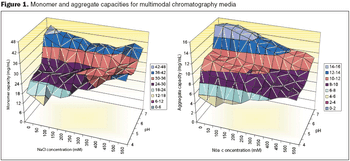
A systematic approach facilitates formulation component selection.

This year's BIO International Convention will be occurring against a backdrop of profound change in our industry.
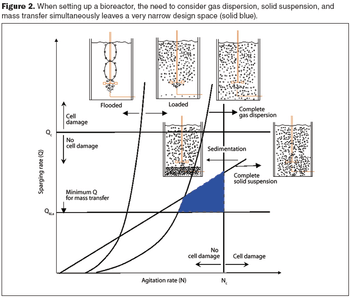
Computational fluid dynamics is a powerful tool to optimize processes.
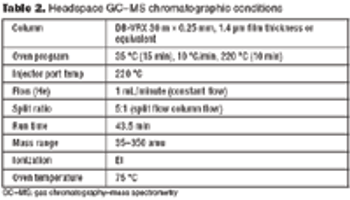
HTPD allows rapid screening of chromatographic parameters.

The FDA is expanding postmarketing safety requirements, despite limited resources to manage these added responsibilities.