
Scale-Up of Human Stem Cells

Scale-Up of Human Stem Cells
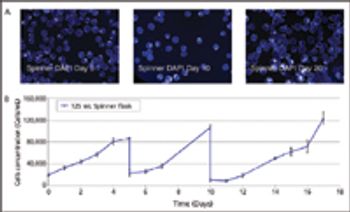
Demonstration of large-scale stem-cell scale-up.
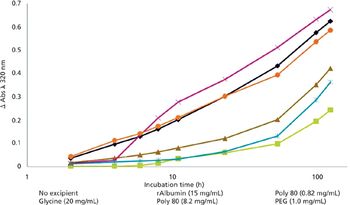
Recombinant albumin can stabilize a drug product and assist in API release.

Does global development have to entail multiple comparability studies?

Social media use raises questions about applying old standards to new information technology.

Key business considerations when developing biosimilar products virtually.

The evolving bio/pharmaceutical business model poses risk for CMOs.

Has the long-awaited guidance answered all of the industry's questions?
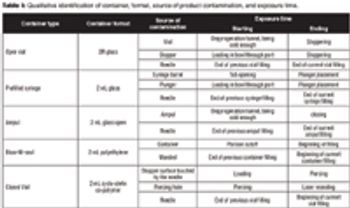
The authors compare the exposure risk from viable particles from the air supply in four well-established aseptic filling technologies.

Focusing on how risk affects the entire organization can improve the business bottom line.

This month, we rewind to an article titled "Demystifying the New Biology."

Key technical considerations when developing a clinical project in the biotech world.