
How to Improve Facility Design and Predict Capacity Mathematical Programming Methods

How to Improve Facility Design and Predict Capacity Mathematical Programming Methods

How this Big Pharma company successfully implemented disposable technologies in its manufacturing plant.

Membrane chromatography ensures purity at high flow rates.

As facility closures and layoffs continue, CRO and CMO executives are hoping for a better year, despite few positive signs.

Trouble at Genzyme and with flu vaccine production illustrates the challenges in producing safe and potent biologics.
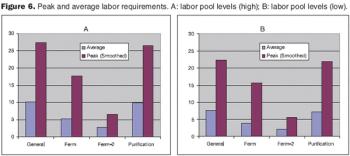
How to improve facility design and predict capacity.

One might look at QbD's plodding growth and conclude that it is never going to make it to graduation.
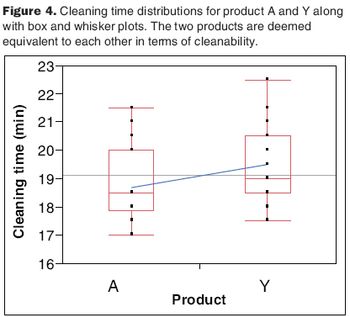
The two-one-sided t-test compares the equivalency of two data sets.